Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining
- PMID: 19133841
- PMCID: PMC2975036
- DOI: 10.1042/BJ20080413
Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining
Abstract
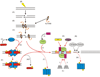
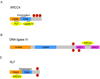
DNA DSBs (double-strand breaks) are considered the most cytotoxic type of DNA lesion. They can be introduced by external sources such as IR (ionizing radiation), by chemotherapeutic drugs such as topoisomerase poisons and by normal biological processes such as V(D)J recombination. If left unrepaired, DSBs can cause cell death. If misrepaired, DSBs may lead to chromosomal translocations and genomic instability. One of the major pathways for the repair of IR-induced DSBs in mammalian cells is NHEJ (non-homologous end-joining). The main proteins required for NHEJ in mammalian cells are the Ku heterodimer (Ku70/80 heterodimer), DNA-PKcs [the catalytic subunit of DNA-PK (DNA-dependent protein kinase)], Artemis, XRCC4 (X-ray-complementing Chinese hamster gene 4), DNA ligase IV and XLF (XRCC4-like factor; also called Cernunnos). Additional proteins, including DNA polymerases mu and lambda, PNK (polynucleotide kinase) and WRN (Werner's Syndrome helicase), may also play a role. In the present review, we will discuss our current understanding of the mechanism of NHEJ in mammalian cells and discuss the roles of DNA-PKcs and DNA-PK-mediated phosphorylation in NHEJ.
Figures



References
-
- Povirk LF. Biochemical mechanisms of chromosomal translocations resulting from DNA double-strand breaks. DNA Repair (Amst) 2006;5:1199–1212. - PubMed
-
- Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 2007;6:923–935. - PubMed
-
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. - PubMed
-
- Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7:861–869. - PubMed
-
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

