Multistep aggregation pathway of human interleukin-1 receptor antagonist: kinetic, structural, and morphological characterization
- PMID: 19134476
- PMCID: PMC2710017
- DOI: 10.1016/j.bpj.2008.10.002
Multistep aggregation pathway of human interleukin-1 receptor antagonist: kinetic, structural, and morphological characterization
Abstract
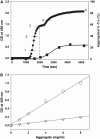
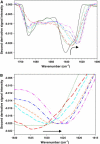
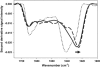
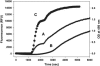
The complex, multistep aggregation kinetic and structural behavior of human recombinant interleukin-1 receptor antagonist (IL-1ra) was revealed and characterized by spectral probes and techniques. At a certain range of protein concentration (12-27 mg/mL) and temperature (44-48 degrees C), two sequential aggregation kinetic transitions emerge, where the second transition is preceded by a lag phase and is associated with the main portion of the aggregated protein. Each kinetic transition is linked to a different type of aggregate population, referred to as type I and type II. The aggregate populations, isolated at a series of time points and analyzed by Fourier-transform infrared spectroscopy, show consecutive protein structural changes, from intramolecular (type I) to intermolecular (type II) beta-sheet formation. The early type I protein spectral change resembles that seen for IL-1ra in the crystalline state. Moreover, Fourier-transform infrared data demonstrate that type I protein assembly alone can undergo a structural rearrangement and, consequently, convert to the type II aggregate. The aggregated protein structural changes are accompanied by the aggregate morphological changes, leading to a well-defined population of interacting spheres, as detected by scanning electron microscopy. A nucleation-driven IL-1ra aggregation pathway is proposed, and assumes two major activation energy barriers, where the second barrier is associated with the type I --> type II aggregate structural rearrangement that, in turn, serves as a pseudonucleus triggering the second kinetic event.
Figures



References
-
- Cleland J.F., Powell M.F., Shire S.J. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit. Rev. Ther. Drug Carrier Syst. 1993;10:307–377. - PubMed
-
- Carpenter J.F., Kendrick B.S., Chang B.S., Manning M.C., Randolph T.W. Inhibition of stress-induced aggregation of protein therapeutics. Methods Enzymol. 1999;309:236–255. - PubMed
-
- Fink A.L. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold. Des. 1998;3:R9–R23. - PubMed
-
- Manning M.C., Patel K., Borchardt R.T. Stability of protein pharmaceuticals. Pharm. Res. 1989;6:903–918. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

