Non-vanillyl resiniferatoxin analogues as potent and metabolically stable transient receptor potential vanilloid 1 agonists
- PMID: 19135377
- PMCID: PMC2798733
- DOI: 10.1016/j.bmc.2008.11.085
Non-vanillyl resiniferatoxin analogues as potent and metabolically stable transient receptor potential vanilloid 1 agonists
Abstract
A series of non-vanillyl resiniferatoxin analogues, having 4-methylsulfonylaminophenyl and fluorophenyl moieties as vanillyl surrogates, have been investigated as ligands for rat TRPV1 heterologously expressed in Chinese hamster ovary cells. Although lacking the metabolically problematic 4-hydroxy substituent on the A-region phenyl ring, the compounds retained substantial agonist potency. Indeed, the 3-methoxy-4-methylsulfonylaminophenyl analog (1) was modestly (2.5-fold) more potent than RTX, with an EC(50)=0.106 nM. Further, it resembled RTX in its kinetics and pattern of stimulation of the levels of intracellular calcium in individual cells, as revealed by imaging. Compound 1 displayed modestly enhanced in vitro stability in rat liver microsomes and in plasma, suggesting that it might be a pharmacokinetically more favorable surrogate of resiniferatoxin. Molecular modeling analyses with selected analogues provide evidence that the conformational differences could affect their binding affinities, especially for the ester versus amide at the B-region.
Figures
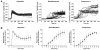





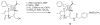
Similar articles
-
Receptor activity and conformational analysis of 5'-halogenated resiniferatoxin analogs as TRPV1 ligands.Bioorg Med Chem Lett. 2011 Jan 1;21(1):299-302. doi: 10.1016/j.bmcl.2010.11.012. Epub 2010 Nov 5. Bioorg Med Chem Lett. 2011. PMID: 21111618 Free PMC article.
-
The carbonate analogues of 5'-halogenated resiniferatoxin as TRPV1 ligands.Eur J Med Chem. 2013 Oct;68:233-43. doi: 10.1016/j.ejmech.2013.07.042. Epub 2013 Aug 9. Eur J Med Chem. 2013. PMID: 23981530 Free PMC article.
-
Structure-activity relationships of the ultrapotent vanilloid resiniferatoxin (RTX): the homovanillyl moiety.Bioorg Med Chem Lett. 2007 Jan 1;17(1):132-5. doi: 10.1016/j.bmcl.2006.09.077. Epub 2006 Sep 29. Bioorg Med Chem Lett. 2007. PMID: 17046253
-
The vanilloid agonist resiniferatoxin for interventional-based pain control.Curr Top Med Chem. 2011;11(17):2171-9. doi: 10.2174/156802611796904942. Curr Top Med Chem. 2011. PMID: 21671877 Free PMC article. Review.
-
Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity.Anesth Analg. 2008 Jul;107(1):271-81. doi: 10.1213/ane.0b013e318162cfa3. Anesth Analg. 2008. PMID: 18635498 Free PMC article. Review.
Cited by
-
Receptor activity and conformational analysis of 5'-halogenated resiniferatoxin analogs as TRPV1 ligands.Bioorg Med Chem Lett. 2011 Jan 1;21(1):299-302. doi: 10.1016/j.bmcl.2010.11.012. Epub 2010 Nov 5. Bioorg Med Chem Lett. 2011. PMID: 21111618 Free PMC article.
-
The carbonate analogues of 5'-halogenated resiniferatoxin as TRPV1 ligands.Eur J Med Chem. 2013 Oct;68:233-43. doi: 10.1016/j.ejmech.2013.07.042. Epub 2013 Aug 9. Eur J Med Chem. 2013. PMID: 23981530 Free PMC article.
-
Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin.Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):E137-45. doi: 10.1073/pnas.1517288113. Epub 2015 Dec 30. Proc Natl Acad Sci U S A. 2016. PMID: 26719417 Free PMC article.
-
The effects of vanilloid analogues structurally related to capsaicin on the transient receptor potential vanilloid 1 channel.Biochem Biophys Rep. 2022 Mar 8;30:101243. doi: 10.1016/j.bbrep.2022.101243. eCollection 2022 Jul. Biochem Biophys Rep. 2022. PMID: 35280525 Free PMC article.
References
-
- Appendino G, Szallasi A. Life Sci. 1997;60:681. - PubMed
-
- Blumberg PM, Szallasi A, Acs G. Resiniferatoxin, an ultrapotent capsaicin Analogue. In: Wood JN, editor. Capsaicin in the study pain. Academic Press; London: 1993. pp. 45–62.
-
- Hergenhahn M, Adolf W, Hecker E. Tetrahedron Lett. 1975;19:1595.
-
- Adolf W, Sorg B, Hergenhahn M, Hecker E. J. Nat. Prod. 1982;45:347. - PubMed
-
- Szallasi A, Blumberg PM. Neuroscience. 1989;30:515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

