Functional selectivity of EGF family peptide growth factors: implications for cancer
- PMID: 19135477
- PMCID: PMC2665203
- DOI: 10.1016/j.pharmthera.2008.11.008
Functional selectivity of EGF family peptide growth factors: implications for cancer
Abstract
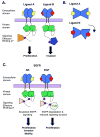
Breast, prostate, pancreatic, colorectal, lung, and head and neck cancers exploit deregulated signaling by ErbB family receptors and their ligands, EGF family peptide growth factors. EGF family members that bind the same receptor are able to stimulate divergent biological responses both in cell culture and in vivo. This is analogous to the functional selectivity exhibited by ligands for G-protein coupled receptors. Here we review this literature and propose that this functional selectivity of EGF family members is due to distinctions in the conformation of the liganded receptor and subsequent differences in the sites of receptor tyrosine phosphorylation and receptor coupling to signaling effectors. We also discuss the roles of divergent ligand activity in establishing and maintaining malignant phenotypes. Finally, we discuss the potential of mutant EGF family ligands as cancer chemotherapeutics targeted to ErbB receptors.
Figures




References
-
- Blackball F, Ranson M, Thatcher N. Where next for gefitinib in patients with lung cancer? Lancet Oncol. 2006;7:499–507. - PubMed
-
- Booth BW, Smith GH. Roles of transforming growth factor-alpha in mammary development and disease. Growth Factors. 2007;25:227–235. - PubMed
-
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. - PubMed
-
- Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

