Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila
- PMID: 19135896
- PMCID: PMC2709536
- DOI: 10.1016/j.cell.2008.12.001
Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila
Abstract
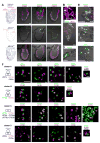
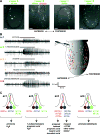
Ionotropic glutamate receptors (iGluRs) mediate neuronal communication at synapses throughout vertebrate and invertebrate nervous systems. We have characterized a family of iGluR-related genes in Drosophila, which we name ionotropic receptors (IRs). These receptors do not belong to the well-described kainate, AMPA, or NMDA classes of iGluRs, and they have divergent ligand-binding domains that lack their characteristic glutamate-interacting residues. IRs are expressed in a combinatorial fashion in sensory neurons that respond to many distinct odors but do not express either insect odorant receptors (ORs) or gustatory receptors (GRs). IR proteins accumulate in sensory dendrites and not at synapses. Misexpression of IRs in different olfactory neurons is sufficient to confer ectopic odor responsiveness. Together, these results lead us to propose that the IRs comprise a novel family of chemosensory receptors. Conservation of IR/iGluR-related proteins in bacteria, plants, and animals suggests that this receptor family represents an evolutionarily ancient mechanism for sensing both internal and external chemical cues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
A new family of odorant receptors in Drosophila.Cell. 2009 Jan 9;136(1):23-5. doi: 10.1016/j.cell.2008.12.031. Cell. 2009. PMID: 19135885
References
-
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. - PubMed
-
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. - PubMed
-
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

