Amygdalar opioids modulate hypothalamic melanocortin-induced anorexia
- PMID: 19136019
- PMCID: PMC4284077
- DOI: 10.1016/j.physbeh.2008.12.007
Amygdalar opioids modulate hypothalamic melanocortin-induced anorexia
Abstract
We wanted to assess the possibility that opioid activity in the central amygdala (CeA) could modulate the feeding inhibition of melanocortin stimulation of the paraventricular hypothalamus (PVN). The melanocortin system is important in both the acute regulation of satiety and feeding behavior and in the integration of long-term appetite signals. Melanotan II (MTII) is a synthetic MC3R and MC4R agonist which reduces food intake when given intracerebroventricularly (ICV) and into the PVN. Tyr-D-Ala-Gly-(me) Phe-Gly-ol (DAMGO), a micro-opioid receptor agonist, increases food intake, while opioid antagonists, like naltrexone (NTX), inhibit food intake after injection into many brain sites involved in appetite regulation, including the CeA. In food-deprived male Sprague-Dawley rats, co-injected intra-PVN MTII partially blocked the orexigenic effect of co-injected intra-CeA DAMGO. Intra-CeA NTX co-injected with intra-PVN MTII reduced food intake significantly more than either alone. NTX administered intra-CeA reduced c-Fos-immunoreactivity (IR) in nucleus accumbens neurons significantly compared to the intra-PVN MTII treated animals, animals co-injected intra-PVN with MTII and intra-CeA with NTX animals, and control animals. Intra-PVN MTII induced c-Fos-IR in significantly more PVN neurons than observed in control animals. Intra-CeA NTX co-injected with intra-PVN MTII induced c-Fos-IR significantly in PVN neurons relative to control and intra-CeA NTX animals. Such data support the significance of opioid action within the CeA as a modulator of the feeding regulation action of melanocortins within the PVN, occurring within the context of a larger appetitive network.
Figures
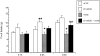
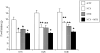
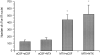

References
-
- Giraudo SQ, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Research. 1998;782:18–23. - PubMed
-
- Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Research. 1998;809:302–306. - PubMed
-
- Giraudo SQ, Kotz CM, Billington CJ, Levine AS. Association between the amygdala and nucleus of the solitary tract in μ-opioid induced feeding in the rat. Brain Research. 1998;802:184–188. - PubMed
-
- Glass MJ, Billington CJ, Levine AS. Naltrexone administered to central nucleus of amygala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integ Comp Physiol. 2000;279:R86–R92. - PubMed
-
- Hansen MJ, Morris MJ. Evidence for an interaction between neuropeptide Y and the melanocortin-4 receptor on feeding in the rat. Neuropharmacology. 2002;42:792–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

