Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro
- PMID: 19136046
- PMCID: PMC2947795
- DOI: 10.1016/j.neuroscience.2008.11.053
Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro
Abstract
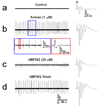
Exposure to organophosphorus nerve agents induces brain seizures, which can cause profound brain damage resulting in death or long-term cognitive deficits. The amygdala and the hippocampus are two of the most seizure-prone brain structures, but their relative contribution to the generation of seizures after nerve agent exposure is unclear. Here, we report that application of 1 muM soman for 30 min, in rat coronal brain slices containing both the hippocampus and the amygdala, produces prolonged synchronous neuronal discharges (10-40 s duration, 1.5-5 min interval of occurrence) resembling ictal activity in the basolateral nucleus of the amygdala (BLA), but only interictal-like activity ("spikes" of 100-250 ms duration; 2-5 s interval) in the pyramidal cell layer of the CA1 hippocampal area. BLA ictal- and CA1 interictal-like activity were synaptically driven, as they were blocked by the AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione. As the expression of the GluR5 subunit of kainate receptors is high in the amygdala, and kainate receptors containing this subunit (GluR5KRs) play an important role in the regulation of neuronal excitability in both the amygdala and the hippocampus, we tested the efficacy of a GluR5KR antagonist against the epileptiform activity induced by soman. The GluR5KR antagonist UBP302 reduced the amplitude of the hippocampal interictal-like spikes, and eliminated the seizure-like discharges in the BLA, or reduced their duration and frequency, with no significant effect on the evoked field potentials. This is the first study reporting in vitro ictal-like activity in response to a nerve agent. Our findings, along with previous literature, suggest that the amygdala may play a more important role than the hippocampus in the generation of seizures following soman exposure, and provide the first evidence that GluR5KR antagonists may be an effective treatment against nerve agent-induced seizures.
Figures


References
-
- Abou-Donia MB. Organophosphorus ester-induced delayed neurotoxicity. Annu Rev Pharmacol Toxicol. 1981;21:511–548. - PubMed
-
- Apland J. Effects of anticonvulsant drugs on soman-induced epileptiform activity in guinea pig hippocampal-entorhinal slices. USAMRICD-TR-01-02. Aberdeen Proving Ground, MD: U.S. Army Medical Research Institute of Chemical Defense; 2001.
-
- Aroniadou-Anderjaska V, Post RM, Rogawski MA, Li H. Input-specific LTP and depotentiation in the basolateral amygdala. NeuroReport. 2001;2:635–640. - PubMed
-
- Aroniadou-Anderjaska V, Qashu F, Braga MF. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: Implications for epilepsy and anxiety disorders. Amino Acids. 2007;32:305–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

