Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS
- PMID: 19136376
- PMCID: PMC2660181
- DOI: 10.1152/ajpgi.90673.2008
Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS
Abstract
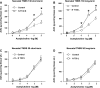
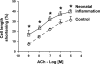
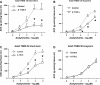
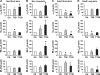
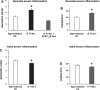
The cellular mechanisms of motility dysfunction in postinfectious irritable bowel syndrome (PI-IBS) are not known. We used a rat model of neonatal inflammation to test the hypothesis that gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in PI-IBS. Mild/moderate or severe inflammation was induced in neonatal and adult rats. Experiments were performed in tissues obtained at 7 days (short term) and 6-8 wk (long term) after the induction of inflammation. Severe inflammation in neonatal rats induced persistent long-term smooth muscle hyperreactivity to acetylcholine (ACh), whereas that in adult rat caused smooth muscle hyporeactivity that showed partial recovery in the long term. Mild/moderate inflammation had no effect in neonatal rats, but it induced smooth muscle hyporeactivity to ACh in adult rats, which recovered fully in the long term. Smooth muscle hyperreactivity to ACh resulted in accelerated colonic transit and increase in defecation rate, whereas hyporeactivity had opposite effects. Smooth muscle hyperreactivity to ACh was associated with increase in transcription rate of key cell-signaling proteins of the excitation-contraction coupling alpha1C subunit of Cav1.2 (L-type) calcium channels, Galphaq, and 20-kDa myosin light chain (MLC20), whereas hyporeactivity was associated with their suppression. Inflammation in adult rats induced classical inflammatory response, which was absent in neonatal rats. Severe neonatal inflammation enhanced plasma norepinephrine and muscularis propria vasoactive intestinal polypeptide in the long term. We conclude that severe, but not mild/moderate, inflammation in a state of immature or impaired stress and immune response systems alters the transcription rate of key cell-signaling proteins of excitation-contraction coupling in colonic circular smooth muscle cells to enhance their contractility and accelerate colonic transit and defecation rate.
Figures




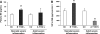
References
-
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 119: 1276–1285, 2000. - PubMed
-
- Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology 127: 524–534, 2004. - PubMed
-
- Bergin AJ, Donnely TC, McKendrik MW, Read NW. Changes in anorectal function in persistent bowel disturbance following salmonella gastroenteritis. Eur J Gastroenterol Hepatol 5: 617–620, 1993.
-
- Camilleri M, Ford MJ. Review article: colonic sensorimotor physiology in health, and its alteration in constipation and diarrhoeal disorders. Aliment Pharmacol Ther 12: 287–302, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

