Re-expression of GATA2 cooperates with peroxisome proliferator-activated receptor-gamma depletion to revert the adipocyte phenotype
- PMID: 19136559
- PMCID: PMC2666598
- DOI: 10.1074/jbc.M809498200
Re-expression of GATA2 cooperates with peroxisome proliferator-activated receptor-gamma depletion to revert the adipocyte phenotype
Abstract
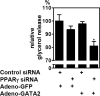
Nuclear peroxisome proliferator-activated receptor-gamma (PPARgamma) is required for adipocyte differentiation, but its role in mature adipocytes is less clear. Here, we report that knockdown of PPARgamma expression in 3T3-L1 adipocytes returned the expression of most adipocyte genes to preadipocyte levels. Consistently, down-regulated but not up-regulated genes showed strong enrichment of PPARgamma binding. Surprisingly, not all adipocyte genes were reversed, and the adipocyte morphology was maintained for an extended period after PPARgamma depletion. To explain this, we focused on transcriptional regulators whose adipogenic regulation was not reversed upon PPARgamma depletion. We identified GATA2, a transcription factor whose down-regulation early in adipogenesis is required for preadipocyte differentiation and whose levels remain low after PPARgamma knockdown. Forced expression of GATA2 in mature adipocytes complemented PPARgamma depletion and impaired adipocyte functionality with a more preadipocyte-like gene expression profile. Ectopic expression of GATA2 in adipose tissue in vivo had a similar effect on adipogenic gene expression. These results suggest that PPARgamma-independent down-regulation of GATA2 prevents reversion of mature adipocytes after PPARgamma depletion.
Figures





References
-
- Rosen, E. D., and MacDougald, O. A. (2006) Nat. Rev. Mol. Cell Biol. 7 885-896 - PubMed
-
- Chawla, A., Schwarz, E. J., Dimaculangan, D. D., and Lazar, M. A. (1994) Endocrinology 135 798-800 - PubMed
-
- Tontonoz, P., Hu, E., Graves, R. A., Budavari, A. I., and Spiegelman, B. M. (1994) Genes Dev. 8 1224-1234 - PubMed
-
- Lehmann, J. M., Moore, L. B., Smith-Oliver, T. A., Wilkison, W. O., Willson, T. M., and Kliewer, S. A. (1995) J. Biol. Chem. 270 12953-12956 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

