Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity
- PMID: 19136649
- PMCID: PMC2661591
- DOI: 10.2337/db08-1644
Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity
Abstract
Objective: To investigate the role of desnutrin in adipose tissue triacylglycerol (TAG) and fatty acid metabolism.
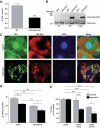
Research design and methods: We generated transgenic mice overexpressing desnutrin (also called adipose triglyceride lipase [ATGL]) in adipocytes (aP2-desnutrin) and also performed adenoviral-mediated overexpression of desnutrin in 3T3-L1CARDelta1 adipocytes.
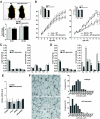
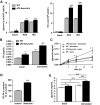
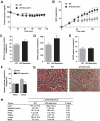
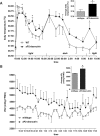
Results: aP2-desnutrin mice were leaner with decreased adipose tissue TAG content and smaller adipocyte size. Overexpression of desnutrin increased lipolysis but did not result in increased serum nonesterified fatty acid levels or ectopic TAG storage. We found increased cycling between diacylglycerol (DAG) and TAG and increased fatty acid oxidation in adipocytes from these mice, as well as improved insulin sensitivity.
Conclusions: We show that by increasing lipolysis, desnutrin overexpression causes reduced adipocyte TAG content and attenuation of diet-induced obesity. Desnutrin-mediated lipolysis promotes fatty acid oxidation and re-esterification within adipocytes.
Figures


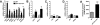
References
-
- Chon S-H, Zhou YX, Dixon JL, Storch J: Developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J Biol Chem 282: 33346– 33357, 2007 - PubMed
-
- Tornqvist H, Belfrage P: Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J Biol Chem 251: 813– 819, 1976 - PubMed
-
- Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW: Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 279: 48968– 48975, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

