Role for MKL1 in megakaryocytic maturation
- PMID: 19136660
- PMCID: PMC2661865
- DOI: 10.1182/blood-2008-09-180596
Role for MKL1 in megakaryocytic maturation
Abstract
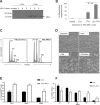
Megakaryoblastic leukemia 1 (MKL1), identified as part of the t(1;22) translocation specific to acute megakaryoblastic leukemia, is highly expressed in differentiated muscle cells and promotes muscle differentiation by activating serum response factor (SRF). Here we show that Mkl1 expression is up-regulated during murine megakaryocytic differentiation and that enforced overexpression of MKL1 enhances megakaryocytic differentiation. When the human erythroleukemia (HEL) cell line is induced to differentiate with 12-O-tetradecanoylphorbol 13-acetate, overexpression of MKL1 results in an increased number of megakaryocytes with a concurrent increase in ploidy. MKL1 overexpression also promotes megakaryocytic differentiation of primary human CD34(+) cells cultured in the presence of thrombopoietin. The effect of MKL1 is abrogated when SRF is knocked down, suggesting that MKL1 acts through SRF. Consistent with these findings in human cells, knockout of Mkl1 in mice leads to reduced platelet counts in peripheral blood, and reduced ploidy in bone marrow megakaryocytes. In conclusion, MKL1 promotes physiologic maturation of human and murine megakaryocytes.
Figures

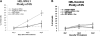


References
-
- Hitzler JK. Acute megakaryoblastic leukemia in Down syndrome. Pediatr Blood Cancer. 2007;49:1066–1069. - PubMed
-
- Simon JH, Tebbi CK, Freeman AI, Brecher ML, Green DM, Sandberg AA. Acute megakaryoblastic leukemia associated with mosaic Down's syndrome. Cancer. 1987;60:2515–2520. - PubMed
-
- Carroll A, Civin C, Schneider N, et al. The t(1;22)p13;q13) is nonrandom and restricted to infants with acute megakaryoblastic leukemia: a Pediatric Oncology Group Study. Blood. 1991;78:748–752. - PubMed
-
- Lion T, Haas OA. Acute megakaryocytic leukemia with the t(1;22)(p13;q13). Leuk Lymphoma. 1993;11:15–20. - PubMed
-
- Lion T, Haas OA, Harbott J, et al. The translocation t(1;22)(p13;q13) is a nonrandom marker specifically associated with acute megakaryocytic leukemia in young children. Blood. 1992;79:3325–3330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32-HL07262/HL/NHLBI NIH HHS/United States
- P30 DK072442/DK/NIDDK NIH HHS/United States
- T32-DK07556/DK/NIDDK NIH HHS/United States
- HL63357/HL/NHLBI NIH HHS/United States
- CA21765/CA/NCI NIH HHS/United States
- T32 DK007556/DK/NIDDK NIH HHS/United States
- T32 HL007262/HL/NHLBI NIH HHS/United States
- R01 DK086267/DK/NIDDK NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- P01 HL063357/HL/NHLBI NIH HHS/United States
- N01-HV-28 186/HV/NHLBI NIH HHS/United States
- DK072442/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

