Type XIV Collagen Regulates Fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE
- PMID: 19136672
- PMCID: PMC2659201
- DOI: 10.1074/jbc.M805582200
Type XIV Collagen Regulates Fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE
Abstract
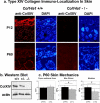
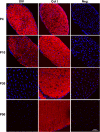
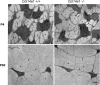
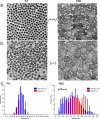
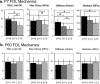
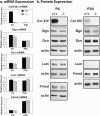
Type XIV collagen is a fibril-associated collagen with an interrupted triple helix. This collagen interacts with the fibril surface and has been implicated as a regulator of fibrillogenesis; however, a specific role has not been elucidated. Functional roles for type XIV collagen were defined utilizing a new type XIV collagen-deficient mouse line. This line was produced using a conventional targeted knock-out approach. Col14a1(-/-) mice were devoid of type XIV collagen, whereas heterozygous mice had reduced synthesis. Both mutant Col14a1 genotypes were viable with a grossly normal phenotype; however, mature skin exhibited altered mechanical properties. Prior to evaluating tendon fibrillogenesis in type XIV collagen-deficient mice, the developmental expression patterns were analyzed in wild-type flexor digitorum longus (FDL) tendons. Analyses of mRNA and protein expression indicated tissue-specific temporal expression that was associated with the early stages in fibrillogenesis. Ultrastructural analyses of wild-type and null tendons demonstrated premature fibril growth and larger fibril diameters in tendons from null mice at postnatal day 4 (P4). However, fibril structure in mature tendons was normal. Biomechanical studies established a direct structure/function relationship with reduced strength in P7-null tendons. However, the biomechanical properties in P60 tendons were comparable in null and wild-type mice. Our results indicate a regulatory function for type XIV collagen in early stages of collagen fibrillogenesis with tissue differences.
Figures

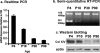
References
-
- van der Rest, M., and Garrone, R. (1991) FASEB J. 5 2814–2823 - PubMed
-
- Kielty, C. M., and Grant, M. E. (2002) in Connective Tissue and Its Heritable Disorders (Royce, P. M., and Steinmann, B., eds) pp. 159–222, Wiley-Liss, New York
-
- Birk, D. E., and Bruckner, P. (2005) in Topics in Current Chemistry: Collagen (Brinckmann, J., Müller, P. K., and Notbohm, H., eds) pp. 185–205, Springer-Verlag, Berlin
-
- Birk, D. E., Nurminskaya, M. V., and Zycband, E. I. (1995) Dev. Dyn. 202 229–243 - PubMed
-
- Birk, D. E., and Mayne, R. (1997) Eur. J. Cell Biol. 72 352–361 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

