AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency
- PMID: 19136964
- PMCID: PMC2863116
- DOI: 10.1038/nm.1904
AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency
Abstract
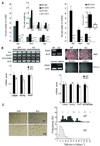
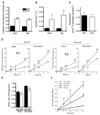
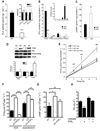
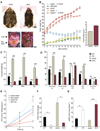
A main function of white adipose tissue is to release fatty acids from stored triacylglycerol for other tissues to use as an energy source. Whereas endocrine regulation of lipolysis has been extensively studied, autocrine and paracrine regulation is not well understood. Here we describe the role of the newly identified major adipocyte phospholipase A(2), AdPLA (encoded by Pla2g16, also called HREV107), in the regulation of lipolysis and adiposity. AdPLA-null mice have a markedly higher rate of lipolysis owing to increased cyclic AMP levels arising from the marked reduction in the amount of adipose prostaglandin E(2) that binds the Galpha(i)-coupled receptor, EP3. AdPLA-null mice have markedly reduced adipose tissue mass and triglyceride content but normal adipogenesis. They also have higher energy expenditure with increased fatty acid oxidation within adipocytes. AdPLA-deficient ob/ob mice remain hyperphagic but lean, with increased energy expenditure, yet have ectopic triglyceride storage and insulin resistance. AdPLA is a major regulator of adipocyte lipolysis and is crucial for the development of obesity.
Figures


References
-
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. - PubMed
-
- Dircks L SH. Acyltransferases of de novo glycerophospholipid biosynthesis. Prog Lipid Res. 1999;38:461–479. - PubMed
-
- Yet SF LS, Hahm YT, Sul HS. Expression and identification of p90 as the murine mitochondrial glycerol-3-phosphate acyltransferase. J Biochem. 1993:9486–9491. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

