Large-scale profiling of protein palmitoylation in mammalian cells
- PMID: 19137006
- PMCID: PMC2775068
- DOI: 10.1038/nmeth.1293
Large-scale profiling of protein palmitoylation in mammalian cells
Abstract
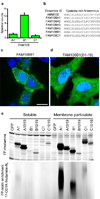
S-palmitoylation is a pervasive post-translational modification required for the trafficking, compartmentalization and membrane tethering of many proteins. We demonstrate that the commercially available compound 17-octadecynoic acid (17-ODYA) can serve as a bioorthogonal, click chemistry probe for in situ labeling, identification and verification of palmitoylated proteins in human cells. We identified approximately 125 predicted palmitoylated proteins, including G proteins, receptors and a family of uncharacterized hydrolases whose plasma membrane localization depends on palmitoylation.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

