Substrate properties influence calcification in valvular interstitial cell culture
- PMID: 19137803
- PMCID: PMC2735090
Substrate properties influence calcification in valvular interstitial cell culture
Abstract
Background and aim of the study: Valvular calcification is an active, cell-mediated process that results in significant morbidity and mortality. In standard culture, valvular interstitial cells (VICs) elicit significant calcification as a result of myofibroblast activation, and this limits their use in characterization studies. The study aim was to identify culturing substrates that would suppress atypical VIC calcification, and to investigate culture substrates representing a more physiological system.
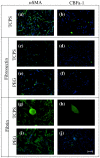
Methods: Several culture platforms were selected to compare and contrast the influence of biochemical and mechanical properties on VIC calcification. Substrates investigated included: tissue culture polystyrene (TCPS), TCPS coated with either fibronectin or fibrin, and an elastic poly(ethylene glycol) (PEG) hydrogel, also with fibronectin or fibrin coupled to the surface. Experiments were repeated with profibrotic growth factor transforming growth factor-beta 1 (TGF-beta1). VIC calcification was characterized by calcific nodule formation, alkaline phosphatase activity and calcium accumulation. Gene and protein expression of alpha smooth muscle actin (aSMA) and core binding factor-1 (CBFa-1) were analyzed with qRT-PCR and immunostaining.
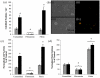
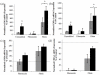
Results: Unmodified TCPS substrates had an innate ability to promote the markers of calcification studied. The addition of TGF-beta1 enhanced levels of all osteoblastic markers studied. When TCPS surfaces were modified with fibronectin, all markers for calcification were repressed, but alphaSMA - a marker for myofibroblastic activity was unchanged. Meanwhile, fibrin-modified TCPS surfaces enhanced calcification over unmodified TCPS substrates. On soft PEG hydrogels, all markers for calcification were repressed, regardless of the surface chemistry, while alphaSMA expression remained unaffected.
Conclusion: Collectively, VIC properties are highly linked to the culture microenvironment. Both, the biochemical and mechanical environment of tissue culture has an effect on the spontaneous calcification of VICs, and may also have a profound effect on their molecular properties, as related to an understanding of the disease process in vivo.
Figures
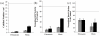
References
-
- Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103(11):1522–1528. - PubMed
-
- Shavelle DM, Otto CM. Cardiology - Chap. 9 Aortic Stenosis. 2 ed Harcourt International; 2004.
-
- Monckeberg JG. Normal histological formation and sclerosis of aorta flaps. Virchows Archiv Fur Pathologische Anatomie Und Physiologie Und Fur Klinische Medizin. 1904;176(3):472–514.
-
- Messier RH, Bass BL, Aly HM, Jones JL, Domkowski PW, Wallace RB, et al. Dual Structural and Functional Phenotypes of the Porcine Aortic-Valve Interstitial Population - Characteristics of the Leaflet Myofibroblast. Journal of Surgical Research. 1994;57(1):1–21. - PubMed
-
- Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Localization and pattern of expression of extracellular matrix components in human heart valves. Journal Of Heart Valve Disease. 2005;14(2):218–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
