Paxillin-Y118 phosphorylation contributes to the control of Src-induced anchorage-independent growth by FAK and adhesion
- PMID: 19138410
- PMCID: PMC2651180
- DOI: 10.1186/1471-2407-9-12
Paxillin-Y118 phosphorylation contributes to the control of Src-induced anchorage-independent growth by FAK and adhesion
Abstract
Background: Focal adhesion kinase (FAK) and Src are protein tyrosine kinases that physically and functionally interact to facilitate cancer progression by regulating oncogenic processes such as cell motility, survival, proliferation, invasiveness, and angiogenesis.
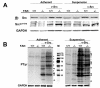
Method: To understand how FAK affects oncogenesis through the phosphorylation of cellular substrates of Src, we analyzed the phosphorylation profile of a panel of Src substrates in parental and v-Src-expressing FAK+/+ and FAK-/- mouse embryo fibroblasts, under conditions of anchorage-dependent (adherent) and -independent (suspension) growth.
Results: Total Src-induced cellular tyrosine phosphorylation as well as the number of phosphotyrosyl substrates was higher in suspension versus adherent cultures. Although the total level of Src-induced cellular phosphorylation was similar in FAK+/+ and FAK-/- backgrounds, the phosphorylation of some substrates was influenced by FAK depending on adherence state. Specifically, in the absence of FAK, Src induced higher phosphorylation of p190RhoGAP, paxillin (poY118) and Crk irrespective of adhesion state, PKC-delta (poY311), connexin-43 (poY265) and Sam68 only under adherent conditions, and p56Dok-2 (poY351) and p120catenin (poY228) only under suspension conditions. In contrast, FAK enhanced the Src-induced phosphorylation of vinculin (poY100 and poY1065) and p130CAS (poY410) irrespective of adherence state, p56Dok-2 (poY351) and p120catenin (poY228) only under adherent conditions, and connexin-43 (poY265), cortactin (poY421) and paxillin (poY31) only under suspension conditions. The Src-induced phosphorylation of Eps8, PLC-gamma 1 and Shc (poY239/poY240) were not affected by either FAK or adherence status. The enhanced anchorage-independent growth of FAK-/-[v-Src] cells was selectively decreased by expression of paxillin Y118F, but not by WT-paxillin, p120catenin Y228F or ShcY239/240F, identifying for the first time a role for paxillinpoY118 in Src-induced anchorage-independent growth. Knockdown of FAK by siRNA in the human colon cancer lines HT-25 and RKO, resulted in increased paxillinpoY118 levels under suspension conditions as well as increased anchorage-independent growth, supporting the notion that FAK attenuates anchorage-independent growth by suppressing adhesion-dependent phosphorylation of paxillin Y118.
Conclusion: These data suggest that phosphorylation of Src substrates is a dynamic process, influenced temporally and spatially by factors such as FAK and adhesion.
Figures





Similar articles
-
Differential effect of the focal adhesion kinase Y397F mutant on v-Src-stimulated cell invasion and tumor growth.J Biomed Sci. 2005;12(4):571-85. doi: 10.1007/s11373-005-7212-5. Epub 2005 Nov 10. J Biomed Sci. 2005. PMID: 16132110
-
v-Src rescues actin-based cytoskeletal architecture and cell motility and induces enhanced anchorage independence during oncogenic transformation of focal adhesion kinase-null fibroblasts.J Biol Chem. 2003 Nov 28;278(48):47946-59. doi: 10.1074/jbc.M302720200. Epub 2003 Sep 18. J Biol Chem. 2003. PMID: 14500722
-
CD151 mediates netrin-1-induced angiogenesis through the Src-FAK-Paxillin pathway.J Cell Mol Med. 2017 Jan;21(1):72-80. doi: 10.1111/jcmm.12939. Epub 2016 Aug 25. J Cell Mol Med. 2017. PMID: 27558487 Free PMC article.
-
Integrin-regulated FAK-Src signaling in normal and cancer cells.Curr Opin Cell Biol. 2006 Oct;18(5):516-23. doi: 10.1016/j.ceb.2006.08.011. Epub 2006 Aug 17. Curr Opin Cell Biol. 2006. PMID: 16919435 Review.
-
Focal Adhesion Kinase (FAK) and c-Src Dependent Signal Transduction in Cell Adhesion.Discov Med. 2024 Oct;36(189):1998-2012. doi: 10.24976/Discov.Med.202436189.184. Discov Med. 2024. PMID: 39463220 Review.
Cited by
-
Light-regulated allosteric switch enables temporal and subcellular control of enzyme activity.Elife. 2020 Sep 23;9:e60647. doi: 10.7554/eLife.60647. Elife. 2020. PMID: 32965214 Free PMC article.
-
The G protein-coupled estrogen receptor-1, GPER-1, promotes fibrillogenesis via a Shc-dependent pathway resulting in anchorage-independent growth.Horm Cancer. 2014 Dec;5(6):390-404. doi: 10.1007/s12672-014-0195-9. Epub 2014 Aug 6. Horm Cancer. 2014. PMID: 25096985 Free PMC article.
-
Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells.Gastroenterology. 2011 Dec;141(6):2140-53. doi: 10.1053/j.gastro.2011.08.038. Epub 2011 Aug 28. Gastroenterology. 2011. PMID: 21878201 Free PMC article.
-
PRC1 promotes cell proliferation and cell cycle progression by regulating p21/p27-pRB family molecules and FAK-paxillin pathway in non-small cell lung cancer.Transl Cancer Res. 2019 Sep;8(5):2059-2072. doi: 10.21037/tcr.2019.09.19. Transl Cancer Res. 2019. PMID: 35116955 Free PMC article.
-
Naphtho[1,2-b]furan-4,5-dione inhibits MDA-MB-231 cell migration and invasion by suppressing Src-mediated signaling pathways.Mol Cell Biochem. 2014 Feb;387(1-2):101-11. doi: 10.1007/s11010-013-1875-4. Epub 2013 Oct 26. Mol Cell Biochem. 2014. PMID: 24162594
References
-
- Abbi S, Guan JL. Focal adhesion kinase: Protein interactions and cellular functions. Histology And Histopathology. 2002;17:1163–1171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

