A low-E magic angle spinning probe for biological solid state NMR at 750 MHz
- PMID: 19138870
- PMCID: PMC2659328
- DOI: 10.1016/j.jmr.2008.12.008
A low-E magic angle spinning probe for biological solid state NMR at 750 MHz
Abstract
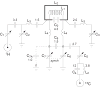
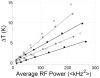
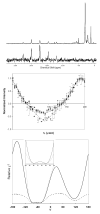
Crossed-coil NMR probes are a useful tool for reducing sample heating for biological solid state NMR. In a crossed-coil probe, the higher frequency (1)H field, which is the primary source of sample heating in conventional probes, is produced by a separate low-inductance resonator. Because a smaller driving voltage is required, the electric field across the sample and the resultant heating is reduced. In this work we describe the development of a magic angle spinning (MAS) solid state NMR probe utilizing a dual resonator. This dual resonator approach, referred to as "low-E," was originally developed to reduce heating in samples of mechanically aligned membranes. The study of inherently dilute systems, such as proteins in lipid bilayers, via MAS techniques requires large sample volumes at high field to obtain spectra with adequate signal-to-noise ratio under physiologically relevant conditions. With the low-E approach, we are able to obtain homogeneous and sufficiently strong radiofrequency fields for both (1)H and (13)C frequencies in a 4mm probe with a (1)H frequency of 750 MHz. The performance of the probe using windowless dipolar recoupling sequences is demonstrated on model compounds as well as membrane-embedded peptides.
Figures









References
-
- Paulson EK, Martin RW, Zilm KW. Cross polarization, radio frequency field homogeneity, and circuit balancing in high field solid state NMR probes. J Magn Reson. 2004;171:314–323. - PubMed
-
- Gor’kov PL, Chekmenev EY, Fu RQ, Hu J, Cross TA, Cotten M, Brey WW. A large volume flat coil probe for oriented membrane proteins. J Magn Reson. 2006;181:9–20. - PubMed
-
- Martin RW, Paulson EK, Zilm KW. Design of a triple resonance magic angle sample spinning probe for high field solid state nuclear magnetic resonance. Rev Sci Instrum. 2003;74:3045–3061.
-
- Stringer JA, Bronnimann CE, Mullen CG, Zhou DH, Stellfox SA, Li Y, Williams EH, Rienstra CM. Reduction of RF-induced sample heating with a scroll coil resonator structure for solid-state NMR probes. J Magn Reson. 2005;173:40–48. - PubMed
-
- de Lacaillerie JBD, Jarry B, Pascui O, Reichert D. “Cooking the sample”: Radiofrequency induced heating during solid-state NMR experiments. Solid State NMR. 2005;28:225–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

