A highly conserved cis-regulatory motif directs differential gonadal synexpression of Dmrt1 transcripts during gonad development
- PMID: 19139075
- PMCID: PMC2655695
- DOI: 10.1093/nar/gkn1065
A highly conserved cis-regulatory motif directs differential gonadal synexpression of Dmrt1 transcripts during gonad development
Abstract
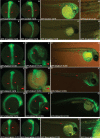
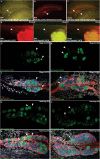
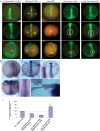
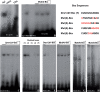
Differential gene expression largely accounts for the coordinated manifestation of the genetic programme underlying embryonic development and cell differentiation. The 3' untranslated region (3'-UTR) of eukaryotic genes can contain motifs involved in regulation of gene expression at the post-transcriptional level. In the 3'-UTR of dmrt1, a key gene that functions in gonad development and differentiation, an 11-bp protein-binding motif was identified that mediates gonad-specific mRNA localization during embryonic and larval development of fish. Mutations that disrupt the 11-bp motif leading to in vitro protein-binding loss and selective transcript stabilization failure indicate a role for this motif in RNA stabilization through protein binding. The sequence motif was found to be conserved in most of the dmrt1 homologous genes from flies to humans suggesting a widespread conservation of this specific mechanism.
Figures
Similar articles
-
A novel evolutionary conserved mechanism of RNA stability regulates synexpression of primordial germ cell-specific genes prior to the sex-determination stage in medaka.PLoS Biol. 2019 Apr 4;17(4):e3000185. doi: 10.1371/journal.pbio.3000185. eCollection 2019 Apr. PLoS Biol. 2019. PMID: 30947255 Free PMC article.
-
Developmentally regulated and non-sex-specific expression of autosomal dmrt genes in embryos of the Medaka fish (Oryzias latipes).Mech Dev. 2004 Jul;121(7-8):997-1005. doi: 10.1016/j.mod.2004.03.018. Mech Dev. 2004. PMID: 15210205
-
Analysis of a novel gene, Sdgc, reveals sex chromosome-dependent differences of medaka germ cells prior to gonad formation.Development. 2014 Sep;141(17):3363-9. doi: 10.1242/dev.106864. Epub 2014 Jul 30. Development. 2014. PMID: 25078651
-
AU-rich elements and associated factors: are there unifying principles?Nucleic Acids Res. 2006 Jan 3;33(22):7138-50. doi: 10.1093/nar/gki1012. Print 2005. Nucleic Acids Res. 2006. PMID: 16391004 Free PMC article. Review.
-
Dmrt1 genes at the crossroads: a widespread and central class of sexual development factors in fish.FEBS J. 2011 Apr;278(7):1010-9. doi: 10.1111/j.1742-4658.2011.08030.x. Epub 2011 Feb 25. FEBS J. 2011. PMID: 21281449 Review.
Cited by
-
Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity.Nat Rev Genet. 2012 Feb 7;13(3):163-74. doi: 10.1038/nrg3161. Nat Rev Genet. 2012. PMID: 22310892 Free PMC article. Review.
-
Divergent expression regulation of gonad development genes in medaka shows incomplete conservation of the downstream regulatory network of vertebrate sex determination.Mol Biol Evol. 2013 Oct;30(10):2328-46. doi: 10.1093/molbev/mst130. Epub 2013 Jul 24. Mol Biol Evol. 2013. PMID: 23883523 Free PMC article.
-
Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics.PLoS One. 2012;7(7):e40701. doi: 10.1371/journal.pone.0040701. Epub 2012 Jul 9. PLoS One. 2012. PMID: 22792396 Free PMC article.
-
A novel evolutionary conserved mechanism of RNA stability regulates synexpression of primordial germ cell-specific genes prior to the sex-determination stage in medaka.PLoS Biol. 2019 Apr 4;17(4):e3000185. doi: 10.1371/journal.pbio.3000185. eCollection 2019 Apr. PLoS Biol. 2019. PMID: 30947255 Free PMC article.
-
Allelic diversification after transposable element exaptation promoted gsdf as the master sex determining gene of sablefish.Genome Res. 2021 Aug;31(8):1366-1380. doi: 10.1101/gr.274266.120. Epub 2021 Jun 28. Genome Res. 2021. PMID: 34183453 Free PMC article.
References
-
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007;87:1–28. - PubMed
-
- Santos AC, Lehmann R. Germ cell specification and migration in Drosophila and beyond. Curr. Biol. 2004;14:R578–R589. - PubMed
-
- McLaren A. Mammalian germ cells: birth, sex, and immortality. Cell Struct. Funct. 2001;26:119–122. - PubMed
-
- Raz E. Guidance of primordial germ cell migration. Curr. Opin. Cell Biol. 2004;16:169–173. - PubMed
-
- Saito D, Morinaga C, Aoki Y, Nakamura S, Mitani H, Furutani-Seiki M, Kondoh H, Tanaka M. Proliferation of germ cells during gonadal sex differentiation in medaka: Insights from germ cell-depleted mutant zenzai. Dev. Biol. 2007;310:280–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

