CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy
- PMID: 19139079
- PMCID: PMC2676090
- DOI: 10.1182/blood-2008-10-186668
CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy
Erratum in
-
Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341-4351.Blood. 2024 Jun 20;143(25):2673. doi: 10.1182/blood.2024025275. Blood. 2024. PMID: 38900482 Free PMC article. No abstract available.
Abstract
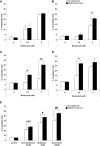
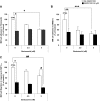
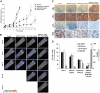
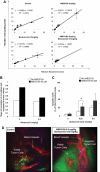
The interaction of multiple myeloma (MM) cells with their microenvironment in the bone marrow (BM) provides a protective environment and resistance to therapeutic agents. We hypothesized that disruption of the interaction of MM cells with their BM milieu would lead to their sensitization to therapeutic agents such as bortezomib, melphalan, doxorubicin, and dexamethasone. We report that the CXCR4 inhibitor AMD3100 induces disruption of the interaction of MM cells with the BM reflected by mobilization of MM cells into the circulation in vivo, with kinetics that differed from that of hematopoietic stem cells. AMD3100 enhanced sensitivity of MM cell to multiple therapeutic agents in vitro by disrupting adhesion of MM cells to bone marrow stromal cells (BMSCs). Moreover, AMD3100 increased mobilization of MM cells to the circulation in vivo, increased the ratio of apoptotic circulating MM cells, and enhanced the tumor reduction induced by bortezomib. Mechanistically, AMD3100 significantly inhibited Akt phosphorylation and enhanced poly(ADP-ribose) polymerase (PARP) cleavage as a result of bortezomib, in the presence of BMSCs in coculture. These experiments provide a proof of concept for the use of agents that disrupt interaction with the microenvironment for enhancement of efficacy of cytotoxic agents in cancer therapy.
Figures

Comment in
-
Priming reloaded?Blood. 2009 Jul 23;114(4):925-6; author reply 926-7. doi: 10.1182/blood-2009-04-217299. Blood. 2009. PMID: 19628718 No abstract available.
References
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. - PubMed
-
- Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Richardson PG. A review of the proteasome inhibitor bortezomib in multiple myeloma. Expert Opin Pharmacother. 2004;5:1321–1331. - PubMed
-
- Richardson PG, Mitsiades CS, Hideshima T, Anderson KC. Novel biological therapies for the treatment of multiple myeloma. Best Pract Res Clin Haematol. 2005;18:619–634. - PubMed
-
- Harousseau JL. Stem cell transplantation in multiple myeloma (0, 1, or 2). Curr Opin Oncol. 2005;17:93–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

