EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology
- PMID: 19139087
- PMCID: PMC2724733
- DOI: 10.1242/jcs.037051
EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology
Abstract
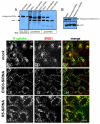
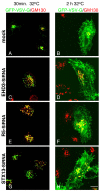
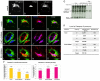
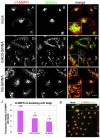
Depletion of EHD3 affects sorting in endosomes by altering the kinetics and route of receptor recycling to the plasma membrane. Here we demonstrate that siRNA knockdown of EHD3, or its interaction partner rabenosyn-5, causes redistribution of sorting nexin 1 (SNX1) to enlarged early endosomes and disrupts transport of internalized Shiga toxin B subunit (STxB) to the Golgi. Moreover, under these conditions, Golgi morphology appears as a series of highly dispersed and fragmented stacks that maintain characteristics of cis-, medial- and trans-Golgi membranes. Although Arf1 still assembled onto these dispersed Golgi membranes, the level of AP-1 gamma-adaptin recruited to the Golgi was diminished. Whereas VSV-G-secretion from the dispersed Golgi remained largely unaffected, the distribution of mannose 6-phosphate receptor (M6PR) was altered: it remained in peripheral endosomes and did not return to the Golgi. Cathepsin D, a hydrolase that is normally transported to lysosomes via an M6PR-dependent pathway, remained trapped at the Golgi. Our findings support a role for EHD3 in regulating endosome-to-Golgi transport, and as a consequence, lysosomal biosynthetic, but not secretory, transport pathways are also affected. These data also suggest that impaired endosome-to-Golgi transport and the resulting lack of recruitment of AP-1 gamma-adaptin to Golgi membranes affect Golgi morphology.
Figures



Similar articles
-
Retromer guides STxB and CD8-M6PR from early to recycling endosomes, EHD1 guides STxB from recycling endosome to Golgi.Traffic. 2012 Aug;13(8):1140-59. doi: 10.1111/j.1600-0854.2012.01374.x. Epub 2012 May 23. Traffic. 2012. PMID: 22540229 Free PMC article.
-
The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network.J Cell Sci. 2007 Jun 15;120(Pt 12):2010-21. doi: 10.1242/jcs.003111. J Cell Sci. 2007. PMID: 17550970
-
Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network.Mol Biol Cell. 2004 Sep;15(9):4011-22. doi: 10.1091/mbc.e03-12-0876. Epub 2004 Jun 23. Mol Biol Cell. 2004. PMID: 15215310 Free PMC article.
-
The GGA proteins: key players in protein sorting at the trans-Golgi network.Eur J Cell Biol. 2004 Jul;83(6):257-62. doi: 10.1078/0171-9335-00374. Eur J Cell Biol. 2004. PMID: 15511083 Review.
-
Targeting the Early Endosome-to-Golgi Transport of Shiga Toxins as a Therapeutic Strategy.Toxins (Basel). 2020 May 22;12(5):342. doi: 10.3390/toxins12050342. Toxins (Basel). 2020. PMID: 32456007 Free PMC article. Review.
Cited by
-
Identification of drug-like molecules targeting the ATPase activity of dynamin-like EHD4.PLoS One. 2024 Jul 29;19(7):e0302704. doi: 10.1371/journal.pone.0302704. eCollection 2024. PLoS One. 2024. PMID: 39074100 Free PMC article.
-
Retromer oligomerization drives SNX-BAR coat assembly and membrane constriction.EMBO J. 2023 Jan 16;42(2):e112287. doi: 10.15252/embj.2022112287. EMBO J. 2023. PMID: 36644906 Free PMC article.
-
The trafficking protein, EHD2, positively regulates cardiac sarcolemmal KATP channel surface expression: role in cardioprotection.FASEB J. 2018 Mar;32(3):1613-1625. doi: 10.1096/fj.201700027R. Epub 2018 Jan 3. FASEB J. 2018. PMID: 29133341 Free PMC article.
-
Neuronal early endosomes require EHD1 for L1/NgCAM trafficking.J Neurosci. 2010 Dec 8;30(49):16485-97. doi: 10.1523/JNEUROSCI.3127-10.2010. J Neurosci. 2010. PMID: 21147988 Free PMC article.
-
Eps15 Homology Domain-containing Protein 3 Regulates Cardiac T-type Ca2+ Channel Targeting and Function in the Atria.J Biol Chem. 2015 May 8;290(19):12210-21. doi: 10.1074/jbc.M115.646893. Epub 2015 Mar 30. J Biol Chem. 2015. PMID: 25825486 Free PMC article.
References
-
- Advani, R. J., Bae, H. R., Bock, J. B., Chao, D. S., Doung, Y. C., Prekeris, R., Yoo, J. S. and Scheller, R. H. (1998). Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273, 10317-10324. - PubMed
-
- Blume, J. J., Halbach, A., Behrendt, D., Paulsson, M. and Plomann, M. (2007). EHD proteins are associated with tubular and vesicular compartments and interact with specific phospholipids. Exp. Cell Res. 313, 219-231. - PubMed
-
- Bolte, S. and Cordelieres, F. P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213-232. - PubMed
-
- Bonifacino, J. S. and Rojas, R. (2006). Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell. Biol. 7, 568-579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

