Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis
- PMID: 19139100
- PMCID: PMC2652269
- DOI: 10.1074/jbc.M807820200
Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis
Erratum in
- J Biol Chem. 2009 Jul 31;284(31):21100. Fulerson, Zachary [corrected to Fulkerson, Zachary]
Abstract
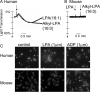
The lipid mediator lysophosphatidic acid (LPA) is a potent regulator of vascular cell function in vitro, but its physiologic role in the cardiovasculature is largely unexplored. To address the role of LPA in regulating platelet function and thrombosis, we investigated the effects of LPA on isolated murine platelets. Although LPA activates platelets from the majority of human donors, we found that treatment of isolated murine platelets with physiologic concentrations of LPA attenuated agonist-induced aggregation. Transgenic overexpression of autotaxin/lysophospholipase D (Enpp2), the enzyme necessary for production of the bulk of biologically active LPA in plasma, elevated circulating LPA levels and induced a bleeding diathesis and attenuation of thrombosis in mice. Intravascular administration of exogenous LPA recapitulated the prolonged bleeding time observed in Enpp2-Tg mice. Enpp2+/- mice, which have approximately 50% normal plasma LPA levels, were more prone to thrombosis. Plasma autotaxin associated with platelets during aggregation and concentrated in arterial thrombus, and activated but not resting platelets bound recombinant autotaxin/lysoPLD in an integrin-dependent manner. These results identify a novel pathway in which LPA production by autotaxin/lysoPLD regulates murine hemostasis and thrombosis and suggest that binding of autotaxin/lysoPLD to activated platelets may provide a mechanism to localize LPA production.
Figures


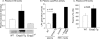

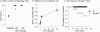

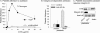
References
-
- Moolenaar, W. H., van der Bend, R. L., van Corven, E. J., Jalink, K., Eichholtz, T., and van Blitterswijk, W. J. (1992) Cold Spring Harbor. Symp. Quant. Biol. 57 163-167 - PubMed
-
- Siess, W., and Tigyi, G. (2004) J. Cell. Biochem. 92 1086-1094 - PubMed
-
- Retzer, M., Siess, W., and Essler, M. (2000) FEBS Lett. 466 70-74 - PubMed
-
- Retzer, M., and Essler, M. (2000) Cell. Signal. 12 645-648 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL070304/HL/NHLBI NIH HHS/United States
- CA096496/CA/NCI NIH HHS/United States
- HL070304/HL/NHLBI NIH HHS/United States
- GM050388/GM/NIGMS NIH HHS/United States
- R01 HL074219/HL/NHLBI NIH HHS/United States
- P30 CA16672/CA/NCI NIH HHS/United States
- S10 RR024598/RR/NCRR NIH HHS/United States
- R01 GM050388/GM/NIGMS NIH HHS/United States
- R01 CA096496/CA/NCI NIH HHS/United States
- HL074219/HL/NHLBI NIH HHS/United States
- HL79396/HL/NHLBI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- R01 HL078663/HL/NHLBI NIH HHS/United States
- HL078663/HL/NHLBI NIH HHS/United States
- P01 CA64602/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

