The Isopeptidase Inhibitor G5 Triggers a Caspase-independent Necrotic Death in Cells Resistant to Apoptosis: A COMPARATIVE STUDY WITH THE PROTEASOME INHIBITOR BORTEZOMIB
- PMID: 19139105
- PMCID: PMC2659195
- DOI: 10.1074/jbc.M806113200
The Isopeptidase Inhibitor G5 Triggers a Caspase-independent Necrotic Death in Cells Resistant to Apoptosis: A COMPARATIVE STUDY WITH THE PROTEASOME INHIBITOR BORTEZOMIB
Abstract
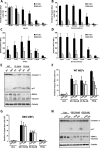
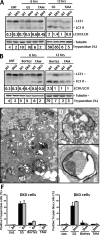
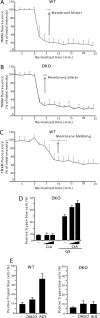
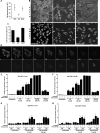
Inhibitors of the ubiquitin-proteasome system (UPSIs) promote apoptosis of cancer cells and show encouraging anti-tumor activities in vivo. In this study, we evaluated the death activities of two different UPSIs: bortezomib and the isopeptidase inhibitor G5. To unveil whether these compounds elicit different types of death, we compared their effect both on apoptosis-proficient wild type mouse embryo fibroblasts and on cells defective for apoptosis (double-deficient Bax/Bak mouse embryo fibroblasts) (double knockout; DKO). We have discovered that (i) both inhibitors induce apoptosis in a Bax and Bak-dependent manner, (ii) both inhibitors elicit autophagy in WT and DKO cells, and (iii) only G5 can kill apoptosis-resistant DKO cells by activating a necrotic response. The induction of necrosis was confirmed by different experimental approaches, including time lapse analysis, HMGB1 release, and electron microscopy studies. Neither treatment with antinecrotic agents, such as antioxidants, poly(ADP-ribose) polymerase and JNK inhibitors, necrostatin, and the intracellular Ca(2+) chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid acetoxymethyl ester, nor overexpression of Bcl-2 and Bcl-xL prevented necrosis induced by G5. This necrotic death is characterized by the absence of protein oxidation and by the rapid cyclosporin A-independent dissipation of the mitochondrial membrane potential. Notably, a peculiar feature of the G5-induced necrosis is an early and dramatic reorganization of the actin cytoskeleton, coupled to an alteration of cell adhesion. The importance of cell adhesion impairment in the G5-induced necrotic death of DKO cells was confirmed by the antagonist effect of the extracellular matrix-adhesive components, collagen and fibronectin.
Figures




Similar articles
-
Characterization of caspase-dependent and caspase-independent deaths in glioblastoma cells treated with inhibitors of the ubiquitin-proteasome system.Mol Cancer Ther. 2009 Nov;8(11):3140-50. doi: 10.1158/1535-7163.MCT-09-0431. Epub 2009 Nov 3. Mol Cancer Ther. 2009. PMID: 19887551
-
Synergistic interaction between the novel histone deacetylase inhibitor ST2782 and the proteasome inhibitor bortezomib in platinum-sensitive and resistant ovarian carcinoma cells.J Inorg Biochem. 2012 Aug;113:94-101. doi: 10.1016/j.jinorgbio.2012.04.007. Epub 2012 Apr 27. J Inorg Biochem. 2012. PMID: 22717676
-
MDM2 antagonist nutlin plus proteasome inhibitor velcade combination displays a synergistic anti-myeloma activity.Cancer Biol Ther. 2010 Jun 1;9(11):936-44. doi: 10.4161/cbt.9.11.11882. Epub 2010 Jun 27. Cancer Biol Ther. 2010. PMID: 20418664
-
Proteasome inhibition in small-cell lung cancer: preclinical rationale and clinical applications.Clin Lung Cancer. 2005 Oct;7 Suppl 2:S67-71. doi: 10.3816/clc.2005.s.012. Clin Lung Cancer. 2005. PMID: 16250931 Review.
-
Altering protein turnover in tumor cells: new opportunities for anti-cancer therapies.Drug Resist Updat. 2005 Dec;8(6):359-68. doi: 10.1016/j.drup.2005.12.001. Epub 2006 Jan 6. Drug Resist Updat. 2005. PMID: 16406769 Review.
Cited by
-
Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib.PLoS One. 2013;8(1):e53263. doi: 10.1371/journal.pone.0053263. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308178 Free PMC article.
-
HMGB1 in health and disease.Mol Aspects Med. 2014 Dec;40:1-116. doi: 10.1016/j.mam.2014.05.001. Epub 2014 Jul 8. Mol Aspects Med. 2014. PMID: 25010388 Free PMC article. Review.
-
Dienone Compounds: Targets and Pharmacological Responses.J Med Chem. 2020 Dec 24;63(24):15075-15093. doi: 10.1021/acs.jmedchem.0c00812. Epub 2020 Nov 4. J Med Chem. 2020. PMID: 33146523 Free PMC article.
-
Isopeptidases in anticancer therapy: looking for inhibitors.Am J Transl Res. 2010 May 10;2(3):235-47. Am J Transl Res. 2010. PMID: 20589164 Free PMC article.
-
The binding landscape of a partially-selective isopeptidase inhibitor with potent pro-death activity, based on the bis(arylidene)cyclohexanone scaffold.Cell Death Dis. 2018 Feb 7;9(2):184. doi: 10.1038/s41419-017-0259-1. Cell Death Dis. 2018. PMID: 29416018 Free PMC article.
References
-
- Ciechanover, A. (2005) Cell Death Differ. 12 1178–1190 - PubMed
-
- Hershko, A., and Ciechanover, A. (1998) Annu. Rev. Biochem. 67 425–479 - PubMed
-
- Amerik, A. Y., and Hochstrasser, M. (2004) Biochim. Biophys. Acta 1695 189–207 - PubMed
-
- Sun, L., and Chen, Z. J. (2004) Curr. Opin. Cell Biol. 16 119–126 - PubMed
-
- Brancolini, C. (2008) Curr. Mol. Pharm. 1 24–37 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

