Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation
- PMID: 19139169
- PMCID: PMC2626670
- DOI: 10.1084/jem.20081445
Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation
Abstract
Novel vaccination strategies against Mycobacterium tuberculosis (MTB) are urgently needed. The use of recombinant MTB antigens as subunit vaccines is a promising approach, but requires adjuvants that activate antigen-presenting cells (APCs) for elicitation of protective immunity. The mycobacterial cord factor Trehalose-6,6-dimycolate (TDM) and its synthetic analogue Trehalose-6,6-dibehenate (TDB) are effective adjuvants in combination with MTB subunit vaccine candidates in mice. However, it is unknown which signaling pathways they engage in APCs and how these pathways are coupled to the adaptive immune response. Here, we demonstrate that these glycolipids activate macrophages and dendritic cells (DCs) via Syk-Card9-Bcl10-Malt1 signaling to induce a specific innate activation program distinct from the response to Toll-like receptor (TLR) ligands. APC activation by TDB and TDM was independent of the C-type lectin receptor Dectin-1, but required the immunoreceptor tyrosine-based activation motif-bearing adaptor protein Fc receptor gamma chain (FcRgamma). In vivo, TDB and TDM adjuvant activity induced robust combined T helper (Th)-1 and Th-17 T cell responses to a MTB subunit vaccine and partial protection against MTB challenge in a Card9-dependent manner. These data provide a molecular basis for the immunostimulatory activity of TDB and TDM and identify the Syk-Card9 pathway as a rational target for vaccine development against tuberculosis.
Figures

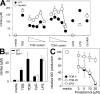
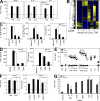

References
-
- Kaufmann, S.H. 2006. Envisioning future strategies for vaccination against tuberculosis. Nat. Rev. Immunol. 6:699–704. - PubMed
-
- Andersen, P., and T.M. Doherty. 2005. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3:656–662. - PubMed
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. - PubMed
-
- Gross, O., A. Gewies, K. Finger, M. Schafer, T. Sparwasser, C. Peschel, I. Forster, and J. Ruland. 2006. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 442:651–656. - PubMed
-
- LeibundGut-Landmann, S., O. Gross, M.J. Robinson, F. Osorio, E.C. Slack, S.V. Tsoni, E. Schweighoffer, V. Tybulewicz, G.D. Brown, J. Ruland, and C. Reis e Sousa. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

