Effect of Chlamydiaphage phiCPG1 on the course of conjunctival infection with "Chlamydia caviae" in guinea pigs
- PMID: 19139194
- PMCID: PMC2643638
- DOI: 10.1128/IAI.01109-08
Effect of Chlamydiaphage phiCPG1 on the course of conjunctival infection with "Chlamydia caviae" in guinea pigs
Abstract
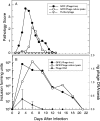
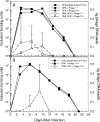
Over the last several years, four different phages of chlamydiae, in addition to a phage associated with Chlamydia psittaci isolated from an ornithosis infection in ducks over 25 years ago, have been described and characterized. While these phages and their chlamydial host specificities have been characterized in vitro, there is virtually nothing known about the interaction of the phage with chlamydiae in their natural animal host. phiCPG1 is a lytic phage specific for "Chlamydia caviae," which is a natural parasite of the guinea pig. In this study, guinea pigs were inoculated in the conjunctiva with suspensions of phiCPG1 and C. caviae and the effect on the development of pathology and on the course of chlamydial infection in the conjunctiva was determined. The presence of phage delayed the appearance of the peak level of chlamydiae in the animal and decreased the pathological response. Evidence for replication of the phage in C. caviae in the conjunctival tissue was observed. Modifying the ratio of phage to chlamydiae altered the course of infection and affected phage replication. There was no evidence for the phage increasing the virulence of C. caviae infection.
Figures

References
-
- Brentlinger, K. L., S. Hafenstein, C. R. Novak, B. A. Fane, R. Borgon, R. McKenna, and M. Agbandje-McKenna. 2002. Microviridae, a family divided: isolation, characterization, and genome sequence of phiMH2K, a bacteriophage of the obligate intracellular parasitic bacterium Bdellovibrio bacteriovorus. J. Bacteriol. 1841089-1094. - PMC - PubMed
-
- Garner, S. A., J. S. Everson, P. R. Lambden, B. A. Fane, and I. N. Clarke. 2004. Isolation, molecular characterisation and genome sequence of a bacteriophage (Chp3) from Chlamydophila pecorum. Virus Genes 28207-214. - PubMed
-
- Hayashi, M., A. Aoyama, J. D. L. Richardson, and M. N. Hayashi. 1988. Biology of the bacteriophage phiX174, p. 1-71. In R. Calendar (ed.), The bacteriophages (the viruses). Plenum Press, New York, NY.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

