Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways
- PMID: 19139264
- PMCID: PMC2615088
- DOI: 10.1083/jcb.200806049
Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways
Abstract
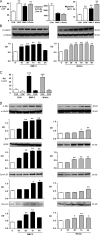
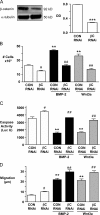
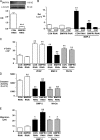
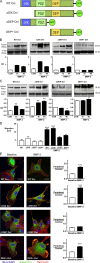
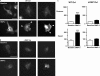
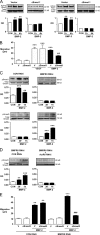
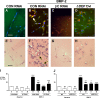
Mutations in bone morphogenetic protein (BMP) receptor II (BMPRII) are associated with pulmonary artery endothelial cell (PAEC) apoptosis and the loss of small vessels seen in idiopathic pulmonary arterial hypertension. Given the low penetrance of BMPRII mutations, abnormalities in other converging signaling pathways may be necessary for disease development. We hypothesized that BMPRII supports normal PAEC function by recruiting Wingless (Wnt) signaling pathways to promote proliferation, survival, and motility. In this study, we report that BMP-2, via BMPRII-mediated inhibition of GSK3-beta, induces beta-catenin (beta-C) accumulation and transcriptional activity necessary for PAEC survival and proliferation. At the same time, BMP-2 mediates phosphorylated Smad1 (pSmad1) or, with loss of BMPRII, pSmad3-dependent recruitment of Disheveled (Dvl) to promote RhoA-Rac1 signaling necessary for motility. Finally, using an angiogenesis assay in severe combined immunodeficient mice, we demonstrate that both beta-C- and Dvl-mediated RhoA-Rac1 activation are necessary for vascular growth in vivo. These findings suggest that the recruitment of both canonical and noncanonical Wnt pathways is required in BMP-2-mediated angiogenesis.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

