The mitogen-activated protein kinase scaffold KSR1 is required for recruitment of extracellular signal-regulated kinase to the immunological synapse
- PMID: 19139278
- PMCID: PMC2648244
- DOI: 10.1128/MCB.01421-08
The mitogen-activated protein kinase scaffold KSR1 is required for recruitment of extracellular signal-regulated kinase to the immunological synapse
Abstract
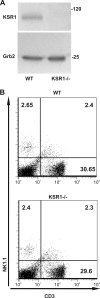
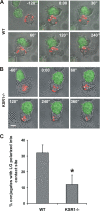
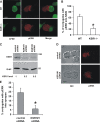
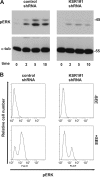
KSR1 is a mitogen-activated protein (MAP) kinase scaffold that enhances the activation of the MAP kinase extracellular signal-regulated kinase (ERK). The function of KSR1 in NK cell function is not known. Here we show that KSR1 is required for efficient NK-mediated cytolysis and polarization of cytolytic granules. Single-cell analysis showed that ERK is activated in an all-or-none fashion in both wild-type and KSR1-deficient cells. In the absence of KSR1, however, the efficiency of ERK activation is attenuated. Imaging studies showed that KSR1 is recruited to the immunological synapse during T-cell activation and that membrane recruitment of KSR1 is required for recruitment of active ERK to the synapse.
Figures




References
-
- Boles, K. S., W. Barchet, T. Diacovo, M. Cella, and M. Colonna. 2005. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 106:779-786. - PubMed
-
- Budhachandra, K., R. K. Brojen Singh, and G. I. Menon. 2008. Microtubule dynamics regulated by stathmin. Comput. Biol. Chem. 32:141-144. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
