Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, but Rec12Spo11 removal is dispensable for other MRN-dependent meiotic functions
- PMID: 19139281
- PMCID: PMC2655602
- DOI: 10.1128/MCB.01182-08
Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, but Rec12Spo11 removal is dispensable for other MRN-dependent meiotic functions
Abstract
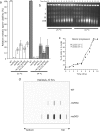
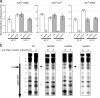
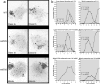
The evolutionarily conserved Mre11/Rad50/Nbs1 (MRN) complex is involved in various aspects of meiosis. Whereas available evidence suggests that the Mre11 nuclease activity might be responsible for Spo11 removal in Saccharomyces cerevisiae, this has not been confirmed experimentally. This study demonstrates for the first time that Mre11 (Schizosaccharomyces pombe Rad32(Mre11)) nuclease activity is required for the removal of Rec12(Spo11). Furthermore, we show that the CtIP homologue Ctp1 is required for Rec12(Spo11) removal, confirming functional conservation between Ctp1(CtIP) and the more distantly related Sae2 protein from Saccharomyces cerevisiae. Finally, we show that the MRN complex is required for meiotic recombination, chromatin remodeling at the ade6-M26 recombination hot spot, and formation of linear elements (which are the equivalent of the synaptonemal complex found in other eukaryotes) but that all of these functions are proficient in a rad50S mutant, which is deficient for Rec12(Spo11) removal. These observations suggest that the conserved role of the MRN complex in these meiotic functions is independent of Rec12(Spo11) removal.
Figures


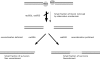
References
-
- Akamatsu, Y., Y. Murayama, T. Yamada, T. Nakazaki, Y. Tsutsui, K. Ohta, and H. Iwasaki. 2008. Molecular characterization of the role of the Schizosaccharomyces pombe nip1+/ctp1+ gene in DNA double-strand break repair in association with the Mre11-Rad50-Nbs1 complex. Mol. Cell. Biol. 283639-3651. - PMC - PubMed
-
- Alani, E., R. Padmore, and N. Kleckner. 1990. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell 61419-436. - PubMed
-
- Bae, S. H., and Y. S. Seo. 2000. Characterization of the enzymatic properties of the yeast dna2 helicase/endonuclease suggests a new model for Okazaki fragment processing. J. Biol. Chem. 27538022-38031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
