Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study
- PMID: 19139364
- PMCID: PMC2677493
- DOI: 10.1212/01.wnl.0000339037.76336.cf
Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study
Abstract
Objectives: To determine whether menopausal hormone therapy (HT) affects regional brain volumes, including hippocampal and frontal regions.
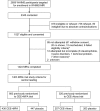
Methods: Brain MRI scans were obtained in a subset of 1,403 women aged 71-89 years who participated in the Women's Health Initiative Memory Study (WHIMS). WHIMS was an ancillary study to the Women's Health Initiative, which consisted of two randomized, placebo-controlled trials: 0.625 mg conjugated equine estrogens (CEE) with or without 2.5 mg medroxyprogesterone acetate (MPA) in one daily tablet. Scans were performed, on average, 3.0 years post-trial for the CEE + MPA trial and 1.4 years post-trial for the CEE-Alone trial; average on-trial follow-up intervals were 4.0 years for CEE + MPA and 5.6 years for CEE-Alone. Total brain, ventricular, hippocampal, and frontal lobe volumes, adjusted for age, clinic site, estimated intracranial volume, and dementia risk factors, were the main outcome variables.
Results: Compared with placebo, covariate-adjusted mean frontal lobe volume was 2.37 cm(3) lower among women assigned to HT (p = 0.004), mean hippocampal volume was slightly (0.10 cm(3)) lower (p = 0.05), and differences in total brain volume approached significance (p = 0.07). Results were similar for CEE + MPA and CEE-Alone. HT-associated reductions in hippocampal volumes were greatest in women with the lowest baseline Modified Mini-Mental State Examination scores (scores <90).
Conclusions: Conjugated equine estrogens with or without MPA are associated with greater brain atrophy among women aged 65 years and older; however, the adverse effects are most evident in women experiencing cognitive deficits before initiating hormone therapy.
Figures
Comment in
-
Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI study.Neurology. 2009 Nov 3;73(18):1514. doi: 10.1212/WNL.0b013e3181bd6a5c. Neurology. 2009. PMID: 19884585 No abstract available.
References
-
- Shumaker SA, Legault C, Thal L, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study—a randomized controlled trial. JAMA 2003;289:2651–2662. - PubMed
-
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947–2958. - PubMed
-
- Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study—a randomized controlled trial. JAMA 2003;289:2663–2672. - PubMed
-
- Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2959–2968. - PubMed
-
- Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative—a randomized trial. JAMA 2003;289:2673–2684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources