Detritivorous crustaceans become herbivores on jasmonate-deficient plants
- PMID: 19139394
- PMCID: PMC2630106
- DOI: 10.1073/pnas.0812182106
Detritivorous crustaceans become herbivores on jasmonate-deficient plants
Abstract
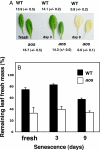
The jasmonate signal pathway is known to control defenses against herbivores, such as leaf eaters (folivores). Does the reach of the pathway extend to defense against other types of animal? Among the arthropods attracted to seed baits placed below flowering Arabidopsis thaliana plants are 2 largely nocturnal isopod crustaceans generally considered as detritivores: Porcellio scaber and Armadillidium vulgare. Parallel laboratory experiments identified the isopods as being capable of predation on intact plants. Isopod feeding was strongly facilitated in jasmonate-deficient Arabidopsis and rice plants. The feeding activity of isopods revealed potentially detritivore-sensitive, jasmonate-protected Achilles' heels in these architecturally different plants (petioles and inflorescence stems in Arabidopsis, and lower stem and mesocotyl in rice). The work addresses the question of what stops 2 detritivores from attacking living plants and provides evidence that it is, in part, the jasmonate signal pathway. Furthermore, senescent leaves from an Arabidopsis jasmonate mutant were consumed more rapidly than senescent wild-type leaves, suggesting that past activity of the jasmonate signal pathway in leaves may slow carbon recycling through detritivory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cyr H, Face ML. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature. 1993;361:148–150.
-
- McNaughton SJ, Oesterheld M, Frank DA, Williams KJ. Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature. 1989;341:142–144. - PubMed
-
- Root RB, Cappuccino N. Patterns in population change and the organization of the insect community associated with goldenrod. Ecol Mono. 1992;62:393–420.
-
- Forget PM, Lambert JE, Hulme PE, Vander Wall SB, editors. Seed Fate: Predation, Dispersal and Seedling Establishment. Wallingford, UK: CABI Publishing; 2005.
-
- Wardle DA. Communities and Ecosystems: Linking the Aboveground and Belowground Components. Princeton: Princeton Univ Press; 2002.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

