TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity
- PMID: 19139399
- PMCID: PMC2621249
- DOI: 10.1073/pnas.0812096106
TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity
Erratum in
-
Correction for Tomasini et al., TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity.Proc Natl Acad Sci U S A. 2018 Sep 11;115(37):E8812. doi: 10.1073/pnas.1814112115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181270 Free PMC article. No abstract available.
Abstract
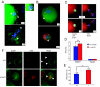
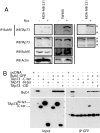
The role of various p73 isoforms in tumorigenesis has been controversial. However, as we have recently shown, the generation of TAp73-deficient (TAp73(-/-)) mice reveals that TAp73 isoforms exert tumor-suppressive functions, indicating an emerging role for Trp-73 in the maintenance of genomic stability. Unlike mice lacking all p73 isoforms, TAp73(-/-) mice show a high incidence of spontaneous tumors. Moreover, TAp73(-/-) mice are infertile and produce oocytes exhibiting spindle abnormalities. These data suggest a link between TAp73 activities and the common molecular machinery underlying meiosis and mitosis. Previous studies have indicated that the spindle assembly checkpoint (SAC) complex, whose activation leads to mitotic arrest, also regulates meiosis. In this study, we demonstrate in murine and human cells that TAp73 is able to interact directly with several partners of the SAC complex (Bub1, Bub3, and BubR1). We also show that TAp73 is involved in SAC protein localization and activities. Moreover, we show that decreased TAp73 expression correlates with increases of SAC protein expression in patients with lung cancer. Our results establish TAp73 as a regulator of SAC responses and indicate that TAp73 loss can lead to mitotic arrest defects. Our data suggest that SAC impairment in the absence of functional TAp73 could explain the genomic instability and increased aneuploidy observed in TAp73-deficient cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yuen KW, Montpetit B, Hieter P. The kinetochore and cancer: what's the connection? Curr Opin Cell Biol. 2005;17:576–582. - PubMed
-
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev. 2001;2:280–291. - PubMed
-
- Thomas NS, et al. Maternal sex chromosome non-disjunction: evidence for X chromosome-specific risk factors. Hum Mol Genet. 2001;10:243–250. - PubMed
-
- Gardner RD, Burke DJ. The spindle checkpoint: two transitions, two pathways. Trends Cell Biol. 2000;10:154–158. - PubMed
-
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

