PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription
- PMID: 19139719
- PMCID: PMC2741690
- DOI: 10.1038/labinvest.2008.168
PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription
Abstract
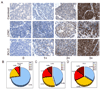
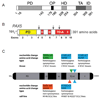
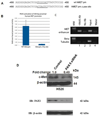
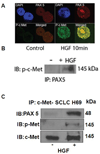
PAX5 is a nuclear transcription factor required for B cell development, and its expression was evaluated in upper aerodigestive malignancies and pancreatic cancer by immunoblotting. The PAX5 protein expression was relatively strong in small-cell lung cancer (SCLC, 11/12); however, its expression was not detected in non-SCLC (NSCLC, n=13), mesothelioma (n=7), pancreatic (n=6), esophageal (n=6) and head and neck cancer cell lines (n=12). In comparison, PAX8 and PAX3 expressions were absent or non-detectable in SCLC cell lines; however, PAX8 was expressed in most of the tested NSCLC cell lines (13/13) and also frequently in all the other cell lines. We also detected frequent expressions of PAX2 and PAX9 protein in the various cell lines. Utilizing neuroendocrine tumor samples, we found that the frequency as well as the average intensity of the expression of PAX5 increased from pulmonary carcinoid (9%, moderate and strong PAX5 expression, n=44), to large-cell neuroendocrine carcinoma (LCNC, 27%, n=11) to SCLC (33%, n=76). FISH analysis revealed no translocations of the PAX5 gene, but polyploidy in some SCLC tumor tissues (6/37). We determined that PAX5 could regulate the transcription of c-Met using luciferase-coupled reporter and chromatin immunoprecipitation analysis. In addition, the phospho-c-Met (active form) and PAX5 were both localized to the same intra-nuclear compartment in hepatocyte growth factor treated SCLC cells and interacted with each other. Finally, we determined the therapeutic translational potential of PAX5 using PAX5 knockdown SCLC cells in conjunction with Topoisomerase 1 (SN38) and c-Met (SU11274) inhibitors. Loss of endogenous PAX5 significantly decreased the viability of SCLC cells, especially when combined with SN38 or SU11274, and maximum effect was seen when both inhibitors were used. Therefore, we propose that PAX5 could be an important regulator of c-Met transcription and a potential target for therapy in SCLC.
Figures




References
-
- Abidoye O, Ferguson MK, Salgia R. Lung carcinoma in African Americans. Nat Clin Pract Oncol. 2007;4(4):118–129. - PubMed
-
- Robson EJD, He S-J, Eccles MR. A panorama of PAX genes in cancer and development. Nature Reviews Cancer. 2006;6:52–62. - PubMed
-
- Cobaleda C, Schebesta A, Delogu A, et al. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8:463–470. - PubMed
-
- Krenacs L, Himmelmann AW, Quintanilla-Martinez L, et al. Transcription factor B-cell-specific activator protein (BSAP) is differentially expressed in B cells and in subsets of B-cell lymphomas. Blood. 1998;A92:1308–1316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

