Clustering T-cell GM1 lipid rafts increases cellular resistance to shear on fibronectin through changes in integrin affinity and cytoskeletal dynamics
- PMID: 19139760
- PMCID: PMC2679097
- DOI: 10.1038/icb.2008.103
Clustering T-cell GM1 lipid rafts increases cellular resistance to shear on fibronectin through changes in integrin affinity and cytoskeletal dynamics
Abstract
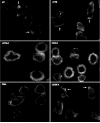
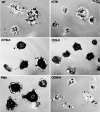
Lipid rafts are small laterally mobile microdomains that are highly enriched in lymphocyte signaling molecules. GM1 gangliosides are a common lipid raft component and have been shown to be important in many T-cell functions. The aggregation of specific GM1 lipid rafts can control many T-cell activation events, including their novel association with T-cell integrins. We found that clustering GM1 lipid rafts can regulate beta1 integrin function. This was apparent through increased resistance to shear flow-dependent detachment of T cells adherent to the alpha4beta1 and alpha5beta1 integrin ligand fibronectin (FN). Adhesion strengthening as a result of clustering GM1 enriched lipid rafts correlated with increased cellular rigidity and morphology through the localization of cortical F-actin, the resistance to shear-induced cell stretching, and an increase in the surface area and symmetry of the contact area between the cell surface and adhesive substrate. Furthermore, clustering GM1 lipid rafts could initiate integrin 'inside-out' signaling mechanisms. This was seen through increased integrin-cytoskeleton associations and enhanced soluble binding of FN and VCAM-1, suggesting the induction of high-affinity integrin conformations. The activation of these adhesion-strengthening characteristics appears to be specific for the aggregation of GM1 lipid rafts as the aggregation of the heterogeneous raft-associated molecule CD59 failed to activate these functions. These findings indicate a novel mechanism to signal to beta1 integrins and to activate adhesion-strengthening processes.
Figures






References
-
- Grabovsky V, Feigelson S, Chen C, Bleijs DA, Peled A, Cinamon G, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506. - PMC - PubMed
-
- Feigelson SW, Grabovsky V, Winter E, Chen LL, Pepinsky RB, Yednock T, et al. The Src kinase p56(lck) up-regulates VLA-4 integrin affinity. Implications for rapid spontaneous and chemokine-triggered T cell adhesion to VCAM-1 and fibronectin. J Biol Chem. 2001;276:13891–901. - PubMed
-
- Laudanna C, Alon R. Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb Haemost. 2006;95:5–11. - PubMed
-
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. - PubMed
-
- Foger N, Marhaba R, Zoller M. Involvement of CD44 in cytoskeleton rearrangement and raft reorganization in T cells. J Cell Sci. 2001;114:1169–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

