The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death
- PMID: 19141071
- PMCID: PMC2884273
- DOI: 10.1111/j.1471-4159.2008.05864.x
The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death
Abstract
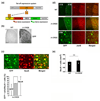
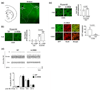
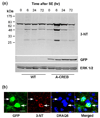
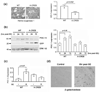
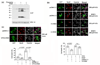
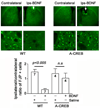
Formation of reactive oxygen and nitrogen species is a precipitating event in an array of neuropathological conditions. In response to excessive reactive oxygen species (ROS) levels, transcriptionally dependent mechanisms drive the up-regulation of ROS scavenging proteins which, in turn, limit the extent of brain damage. Here, we employed a transgenic approach in which cAMP-response element binding protein (CREB)-mediated transcription is repressed (via A-CREB) to examine the contribution of the CREB/cAMP response element pathway to neuroprotection and its potential role in limiting ROS toxicity. Using the pilocarpine-evoked repetitive seizure model, we detected a marked enhancement of cell death in A-CREB transgenic mice. Paralleling this, there was a dramatic increase in tyrosine nitration (a marker of reactive species formation) in A-CREB transgenic mice. In addition, inducible expression of peroxisome proliferator-activated receptor gamma coactivator-1alpha was diminished in A-CREB transgenic mice, as was activity of complex I of the mitochondrial electron transport chain. Finally, the neuroprotective effect of brain-derived neurotrophic factor (BDNF) against ROS-mediated cell death was abrogated by disruption of CREB-mediated transcription. Together, these data both extend our understanding of CREB functionality and provide in vivo validation for a model in which CREB functions as a pivotal upstream integrator of neuroprotective signaling against ROS-mediated cell death.
Figures

References
-
- Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. - PubMed
-
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J. Neural. Transm. Suppl. 2000;59:133–154. - PubMed
-
- Applied Biosystems. Essentials of Real Time PCR. 2003
-
- Aracena P, Aguirre P, Muñoz P, Núñez MT. Iron and glutathione at the crossroad of redox metabolism in neurons. Biol. Res. 2006;39:157–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

