The structure of the MAP2K MEK6 reveals an autoinhibitory dimer
- PMID: 19141286
- PMCID: PMC3689539
- DOI: 10.1016/j.str.2008.11.007
The structure of the MAP2K MEK6 reveals an autoinhibitory dimer
Abstract
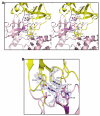
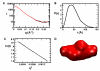
MAP2Ks are dual-specificity protein kinases functioning at the center of three-tiered MAP kinase modules. The structure of the kinase domain of the MAP2K MEK6 with phosphorylation site mimetic aspartic acid mutations (MEK6/DeltaN/DD) has been solved at 2.3 angstroms resolution. The structure reveals an autoinhibited elongated ellipsoidal dimer. The enzyme adopts an inactive conformation, based upon structural queues, despite the phosphomimetic mutations. Gel filtration and small-angle X-ray scattering analysis confirm that the crystallographically observed ellipsoidal dimer is a feature of MEK6/DeltaN/DD and full-length unphosphorylated wild-type MEK6 in solution. The interface includes the phosphate binding ribbon of each subunit, part of the activation loop, and a rare "arginine stack" between symmetry-related arginine residues in the N-terminal lobe. The autoinhibited structure likely confers specificity on active MAP2Ks. The dimer may also serve the function in unphosphorylated MEK6 of preventing activation loop phosphorylation by inappropriate kinases.
Figures



References
-
- Bellon S, Fitzgibbon MJ, Fox T, Hsiao HM, Wilson KP. The structure of phosphorylated p38gamma is monomeric and reveals a conserved activation-loop conformation. Structure. 1999;7:1057–1065. - PubMed
-
- Braun S, Raymond WE, Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984;259:2051–2054. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

