Role of regulatory T cells in coronavirus-induced acute encephalitis
- PMID: 19141357
- PMCID: PMC2684864
- DOI: 10.1016/j.virol.2008.12.014
Role of regulatory T cells in coronavirus-induced acute encephalitis
Abstract
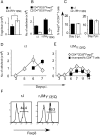
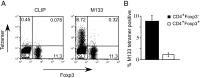
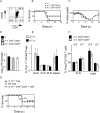
C57BL/6 mice infected with mouse hepatitis virus, strain JHM (JHMV) develop a rapidly fatal acute encephalitis. Previously, we showed that this disease is partially CD4 T cell-mediated since infection with a recombinant JHMV (rJ) mutated in only a single immunodominant CD4 T cell epitope (epitope M133, rJ.M(Y135Q)) results in a nonlethal disease. Increased mortality correlated with a greater number of JHMV-specific CD4 T cells in the brains of rJ compared to rJ.M(Y135Q)-infected mice. Here, we extend these results to show that the diminished number of virus-specific T cells correlates with a reduced cytokine/chemokine response in the infected brain. We also show that regulatory CD4 T cells (Tregs) are critical for mild disease in rJ.M(Y135Q)-infected mice because their depletion results in increased mortality. Further, a relative paucity of Tregs characterizes lethal infection because adoptive transfer of Tregs into rJ-infected mice increases survival from 0% to 50%. These results support the notion that clinical disease in coronavirus-induced acute encephalitis results from a balance between factors critical for virus clearance, such as virus-specific effector T cells and anti-inflammatory elements, such as Tregs. These findings also show that unlike chronic infections, in which an excessive number of Tregs contributes to pathogen persistence, Tregs in the setting of acute encephalitis may help to limit immunopathological disease without delaying virus clearance.
Figures


References
-
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev., Immunol. 2007;7(11):875–888. - PubMed
-
- Belkaid Y., Rouse B.T. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005;6(4):353–360. - PubMed
-
- Couper K.N., Blount D.G., Riley E.M. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180(9):5771–5777. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

