Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9
- PMID: 19141565
- PMCID: PMC2656428
- DOI: 10.1189/jlb.1008588
Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9
Abstract
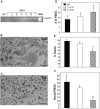
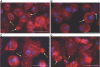
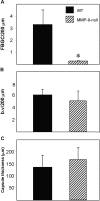
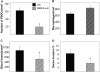
Macrophages undergo fusion to form multinucleated giant cells in several pathologic conditions, including the foreign body response (FBR). We detected high levels of matrix metalloproteinase (MMP)-9 during macrophage fusion in vitro and in foreign body giant cells (FBGCs) in vivo. Wild-type (WT) bone marrow-derived macrophages were induced to fuse with IL-4 in the presence of MMP-9 function-blocking antibodies and displayed reduced fusion. A similar defect, characterized by delayed shape change and abnormal morphology, was observed in MMP-9 null macrophages. Analysis of the FBR in MMP-9 null mice was then pursued to evaluate the significance of these findings. Specifically, mixed cellulose ester disks and polyvinyl alcohol sponges were implanted s.c. in MMP-9 null and WT mice and excised 2-4 weeks later. Histochemical and immunohistochemical analyses indicated equal macrophage recruitment between MMP-9 null and WT mice, but FBGC formation was compromised in the former. In addition, MMP-9 null mice displayed abnormalities in extracellular matrix assembly and angiogenesis. Consistent with a requirement for MMP-9 in fusion, we also observed reduced MMP-9 levels in MCP-1 null macrophages, previously shown to be defective in FBGC formation. Collectively, our studies show abnormalities in MMP-9 null mice during the FBR and suggest a role for MMP-9 in macrophage fusion.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

