Is left-right asymmetry a form of planar cell polarity?
- PMID: 19141667
- PMCID: PMC2687587
- DOI: 10.1242/dev.015974
Is left-right asymmetry a form of planar cell polarity?
Abstract
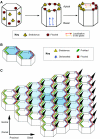
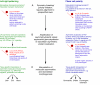
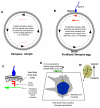
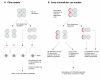
Consistent left-right (LR) patterning is a clinically important embryonic process. However, key questions remain about the origin of asymmetry and its amplification across cell fields. Planar cell polarity (PCP) solves a similar morphogenetic problem, and although core PCP proteins have yet to be implicated in embryonic LR asymmetry, studies of mutations affecting planar polarity, together with exciting new data in cell and developmental biology, provide a new perspective on LR patterning. Here we propose testable models for the hypothesis that LR asymmetry propagates as a type of PCP that imposes coherent orientation onto cell fields, and that the cue that orients this polarization is a chiral intracellular structure.
Figures

References
-
- Adler, P. N. (2002). Planar signaling and morphogenesis in Drosophila. Dev. Cell 2, 525-535. - PubMed
-
- Adler, P. N., Charlton, J. and Liu, J. (1998). Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development 125, 959-968. - PubMed
-
- Adler, P. N., Taylor, J. and Charlton, J. (2000). The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech. Dev. 96, 197-207. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

