Characterization of the A2B adenosine receptor from mouse, rabbit, and dog
- PMID: 19141710
- PMCID: PMC2670590
- DOI: 10.1124/jpet.108.148270
Characterization of the A2B adenosine receptor from mouse, rabbit, and dog
Abstract
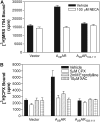
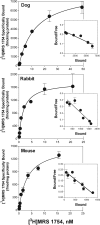
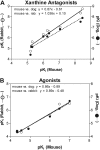
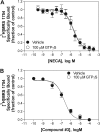
We have cloned and pharmacologically characterized the A(2B) adenosine receptor (AR) from the dog, rabbit, and mouse. The full coding regions of the dog and mouse A(2B)AR were obtained by reverse transcriptase-polymerase chain reaction, and the rabbit A(2B)AR cDNA was obtained by screening a rabbit brain cDNA library. It is noteworthy that an additional clone was isolated by library screening that was identical in sequence to the full-length rabbit A(2B)AR, with the exception of a 27-base pair deletion in the region encoding amino acids 103 to 111 (A(2B)AR(103-111)). This 9 amino acid deletion is located in the second intracellular loop at the only known splice junction of the A(2B)AR and seems to result from the use of an additional 5' donor site found in the rabbit and dog but not in the human, rat, or mouse sequences. [(3)H]3-Isobutyl-8-pyrrolidinoxanthine and 8-[4-[((4-cyano-[2,6-(3)H]-phenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine ([(3)H]MRS 1754) bound with high affinity to membranes prepared from human embryonic kidney (HEK) 293 cells expressing mouse, rabbit, and dog A(2B)ARs. Competition binding studies performed with a panel of agonist (adenosine and 2-amino-3,5-dicyano-4-phenylpyridine analogs) and antagonist ligands identified similar potency orders for the A(2B)AR orthologs, although most xanthine antagonists displayed lower binding affinity for the dog A(2B)AR compared with A(2B)ARs from rabbit and mouse. No specific binding could be detected with membranes prepared from HEK 293 cells expressing the rabbit A(2B)AR(103-111) variant. Furthermore, the variant failed to stimulate adenylyl cyclase or calcium mobilization. We conclude that significant differences in antagonist pharmacology of the A(2B)AR exist between species and that some species express nonfunctional variants of the A(2B)AR due to "leaky" splicing.
Figures





References
-
- Auchampach JA, Jin X, Wan TC, Caughey GH, and Linden J (1997) Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol 52 846-860. - PubMed
-
- Beukers MW, Chang LC, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Spanjersberg RF, Brussee J, and IJzerman AP (2004) New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J Med Chem 47 3707-3709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

