Quantification of phase I/II metabolizing enzyme gene expression and polycyclic aromatic hydrocarbon-DNA adduct levels in human prostate
- PMID: 19143007
- PMCID: PMC2647988
- DOI: 10.1002/pros.20898
Quantification of phase I/II metabolizing enzyme gene expression and polycyclic aromatic hydrocarbon-DNA adduct levels in human prostate
Abstract
Background: Studies of migrant populations suggest that dietary and/or environmental factors play a crucial role in the etiology of prostatic adenocarcinoma (CaP). The human prostate consists of the peripheral zone (PZ), transition zone (TZ), and central zone (CZ); CaP occurs most often in the PZ.
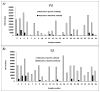
Methods: To investigate the notion that an underlying differential expression of phase I/II genes, and/or the presence of polycyclic aromatic hydrocarbon (PAH)-DNA adducts might explain the elevated PZ susceptibility, we examined prostate tissues (matched tissue sets consisting of PZ and TZ) from men undergoing radical retropubic prostatectomy for CaP (n = 26) or cystoprostatectomy (n = 1). Quantitative gene expression analysis was employed for cytochrome P450 (CYP) isoforms CYP1A1, CYP1B1, and CYP1A2, as well as N-acetyltransferase 1 and 2 (NAT1 and NAT2) and catechol-O-methyl transferase (COMT).
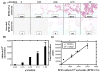
Results: CYP1B1, NAT1, and COMT were expressed in all tissue sets; levels of CYP1B1 and NAT1 were consistently higher in the PZ compared to TZ. Immunohistochemistry confirmed the presence of CYP1B1 (nuclear-associated and primarily in basal epithelial cells) and NAT1. Normal tissue from 23 of these aforementioned 27 matched tissue sets was analyzed for PAH-DNA adduct levels using antiserum elicited against DNA modified with r7,t8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydro-benzo[a]pyrene (BPDE). PAH-DNA adduct levels were highest in glandular epithelial cells, but a comparison of PZ and TZ showed no significant differences.
Conclusion: Although expression of activating and/or detoxifying enzymes may be higher in the PZ, PAH-DNA adduct levels appear to be similar in both zones. Therefore, factors other than PAH-DNA adducts may be responsible for promotion of tumor formation in the human prostate.
(c) 2009 Wiley-Liss, Inc.
Conflict of interest statement
Figures



References
-
- Cancer Research UK. http://info.cancerresearchuk.org/cancerstats/types/prostate/
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. - PubMed
-
- Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005;41:834–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

