Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium
- PMID: 19143467
- PMCID: PMC2621263
- DOI: 10.1371/journal.pmed.0060001
Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium
Abstract
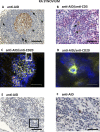
Background: Follicular structures resembling germinal centres (GCs) that are characterized by follicular dendritic cell (FDC) networks have long been recognized in chronically inflamed tissues in autoimmune diseases, including the synovium of rheumatoid arthritis (RA). However, it is debated whether these ectopic structures promote autoimmunity and chronic inflammation driving the production of pathogenic autoantibodies. Anti-citrullinated protein/peptide antibodies (ACPA) are highly specific markers of RA, predict a poor prognosis, and have been suggested to be pathogenic. Therefore, the main study objectives were to determine whether ectopic lymphoid structures in RA synovium: (i) express activation-induced cytidine deaminase (AID), the enzyme required for somatic hypermutation and class-switch recombination (CSR) of Ig genes; (ii) support ongoing CSR and ACPA production; and (iii) remain functional in a RA/severe combined immunodeficiency (SCID) chimera model devoid of new immune cell influx into the synovium.
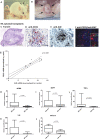
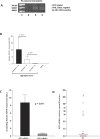
Methods and findings: Using immunohistochemistry (IHC) and quantitative Taqman real-time PCR (QT-PCR) in synovial tissue from 55 patients with RA, we demonstrated that FDC+ structures invariably expressed AID with a distribution resembling secondary lymphoid organs. Further, AID+/CD21+ follicular structures were surrounded by ACPA+/CD138+ plasma cells, as demonstrated by immune reactivity to citrullinated fibrinogen. Moreover, we identified a novel subset of synovial AID+/CD20+ B cells outside GCs resembling interfollicular large B cells. In order to gain direct functional evidence that AID+ structures support CSR and in situ manufacturing of class-switched ACPA, 34 SCID mice were transplanted with RA synovium and humanely killed at 4 wk for harvesting of transplants and sera. Persistent expression of AID and Igamma-Cmu circular transcripts (identifying ongoing IgM-IgG class-switching) was observed in synovial grafts expressing FDCs/CD21L. Furthermore, synovial mRNA levels of AID were closely associated with circulating human IgG ACPA in mouse sera. Finally, the survival and proliferation of functional B cell niches was associated with persistent overexpression of genes regulating ectopic lymphoneogenesis.
Conclusions: Our demonstration that FDC+ follicular units invariably express AID and are surrounded by ACPA-producing plasma cells provides strong evidence that ectopic lymphoid structures in the RA synovium are functional and support autoantibody production. This concept is further confirmed by evidence of sustained AID expression, B cell proliferation, ongoing CSR, and production of human IgG ACPA from GC+ synovial tissue transplanted into SCID mice, independently of new B cell influx from the systemic circulation. These data identify AID as a potential therapeutic target in RA and suggest that survival of functional synovial B cell niches may profoundly influence chronic inflammation, autoimmunity, and response to B cell-depleting therapies.
Conflict of interest statement
Figures



References
-
- Callahan LF, Pincus T. Mortality in the rheumatic diseases. Arthritis Care Res. 1995;8:229–241. - PubMed
-
- Waaler E. The occurrence of a factor in the human serum activating the specific agglutination of sheep blood corpuscles. Acta Pathol Microbiol Scand. 1940;17:172–188. - PubMed
-
- Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–594. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

