The influence of catalysis on mad2 activation dynamics
- PMID: 19143472
- PMCID: PMC2621267
- DOI: 10.1371/journal.pbio.1000010
The influence of catalysis on mad2 activation dynamics
Abstract
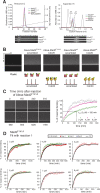
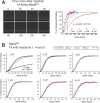
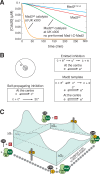
Mad2 is a key component of the spindle assembly checkpoint, a safety device ensuring faithful sister chromatid separation in mitosis. The target of Mad2 is Cdc20, an activator of the anaphase-promoting complex/cyclosome (APC/C). Mad2 binding to Cdc20 is a complex reaction that entails the conformational conversion of Mad2 from an open (O-Mad2) to a closed (C-Mad2) conformer. Previously, it has been hypothesized that the conversion of O-Mad2 is accelerated by its conformational dimerization with C-Mad2. This hypothesis, known as the Mad2-template hypothesis, is based on the unproven assumption that the natural conversion of O-Mad2 required to bind Cdc20 is slow. Here, we provide evidence for this fundamental assumption and demonstrate that conformational dimerization of Mad2 accelerates the rate of Mad2 binding to Cdc20. On the basis of our measurements, we developed a set of rate equations that deliver excellent predictions of experimental binding curves under a variety of different conditions. Our results strongly suggest that the interaction of Mad2 with Cdc20 is rate limiting for activation of the spindle checkpoint. Conformational dimerization of Mad2 is essential to accelerate Cdc20 binding, but it does not modify the equilibrium of the Mad2:Cdc20 interaction, i.e., it is purely catalytic. These results surpass previously formulated objections to the Mad2-template model and predict that the release of Mad2 from Cdc20 is an energy-driven process.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures



References
-
- Nasmyth K. Separating sister chromatids. Trends Biochem Sci. 1999;24:98–104. - PubMed
-
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. - PubMed
-
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

