Impact of age, leukocyte count and day 21-bone marrow response to chemotherapy on the long-term outcome of children with philadelphia chromosome-positive acute lymphoblastic leukemia in the pre-imatinib era: results of the FRALLE 93 study
- PMID: 19144139
- PMCID: PMC2629767
- DOI: 10.1186/1471-2407-9-14
Impact of age, leukocyte count and day 21-bone marrow response to chemotherapy on the long-term outcome of children with philadelphia chromosome-positive acute lymphoblastic leukemia in the pre-imatinib era: results of the FRALLE 93 study
Abstract
Background: We explored the heterogeneity of philadelphia chromosome-positive acute lymphoblastic leukemia (Ph1-ALL) in a study of the effect of early features on prognosis in children. Here we report the long-term results of the FRALLE 93 study conducted in the era before the use of tyrosine kinase inhibitors.
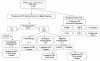
Methods: Between 1993 and 1999, 36 children with Ph1-ALL were enrolled into the FRALLE 93 protocol. After conventional four-drug induction, children were stratified by availability of an HLA-matched sibling.
Results: Complete remission (CR) was observed in 26 children (72%), of which 13 underwent allogeneic bone marrow transplantation (BMT). Thirty-one children were good responders to prednisone, defined on day 8, and 21 were good responders to chemotherapy, defined by day-21 bone marrow (M1). Overall five-year disease-free survival (DFS) was 42 +/- 9.7%. Based on multivariate analysis, two groups showed marked differences in five-year outcome: children with age<10, leukocyte count <100,000/mm3 and day-21 M1 marrow had a more favorable prognosis (14 pts: 100% CR, event free survival [EFS]: 57%, overall survival [OS]: 79%), than the high-risk group (22 patients: 55% CR, EFS: 18%, OS: 27%) (p < 0.005). We also observed a non statistically significant difference (p = 0.14) in outcome between these groups for transplanted patients (5-year DFS: 83 +/- 14% and 33 +/- 15%, respectively).
Conclusion: Age, leukocyte count and early response to treatment defined by the D21 bone marrow response provide an accurate model for outcome prediction. The combination of available tools such as minimal residual disease assessment with determination of these simple factors could be useful for refining indications for BMT in the current era of tyrosine-kinase inhibitor-based therapy.
Figures

References
-
- Roy A, Bradburn M, Moorman A, Burrett J. Early response to induction is predictive of survival in chilhood philadelphia chromosome positive acute lymphoblastic leukemia: results of the Medical Research Council ALL 97 trial. Br J Haematol. 2005;129:35–44. doi: 10.1111/j.1365-2141.2005.05425.x. - DOI - PubMed
-
- Schultz K, Pullen D, Sather H, Shuster J, Devidas M, Borowitz M, Carroll A, Heerema N, Rubnitz J, Loh M, Raetz E, Winick N, Hunger S, Carroll W, Gaynon P, Camitta B. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. - DOI - PMC - PubMed
-
- Arico M, Valsecchi M, Camitta B, Schrappe M, Chessells J, Baruchel A, Gaynon P, Silverman L, Janka-Schaub G, Kamps W, Pui C-H, Masera G. Outcome of treatment in children with philadelphia chromosome positive childhood acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. - DOI - PubMed
-
- Oudot C, Auclerc MF, Levy M, Porcher R, Piguet C, Perel Y, Gandemer V, Debre M, Vermylen C, Pautard B, Berger C, Schmitt C, Leblanc T, Cayuela JM, Socie G, Michel G, Leverger G, Baruchel A. Prognostic factors for leukemic induction failure in children with acute lymphoblastic leukemia and outcome after salvage therapy: the FRALLE 93 study. J Clin Oncol. 2008;26(9):1496–1503. doi: 10.1200/JCO.2007.12.2820. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

