Lentivirus-mediated transgene delivery to the hippocampus reveals sub-field specific differences in expression
- PMID: 19144149
- PMCID: PMC2647928
- DOI: 10.1186/1471-2202-10-2
Lentivirus-mediated transgene delivery to the hippocampus reveals sub-field specific differences in expression
Abstract
Background: In the adult hippocampus, the granule cell layer of the dentate gyrus is a heterogeneous structure formed by neurons of different ages, morphologies and electrophysiological properties. Retroviral vectors have been extensively used to transduce cells of the granule cell layer and study their inherent properties in an intact brain environment. In addition, lentivirus-based vectors have been used to deliver transgenes to replicative and non-replicative cells as well, such as post mitotic neurons of the CNS. However, only few studies have been dedicated to address the applicability of these widespread used vectors to hippocampal cells in vivo. Therefore, the aim of this study was to extensively characterize the cell types that are effectively transduced in vivo by VSVg-pseudotyped lentivirus-based vectors in the hippocampus dentate gyrus.
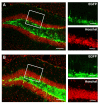
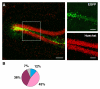
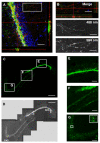
Results: In the present study we used Vesicular Stomatitis Virus G glycoprotein-pseudotyped lentivirual vectors to express EGFP from three different promoters in the mouse hippocampus. In contrast to lentiviral transduction of pyramidal cells in CA1, we identified sub-region specific differences in transgene expression in the granule cell layer of the dentate gyrus. Furthermore, we characterized the cell types transduced by these lentiviral vectors, showing that they target primarily neuronal progenitor cells and immature neurons present in the sub-granular zone and more immature layers of the granule cell layer.
Conclusion: Our observations suggest the existence of intrinsic differences in the permissiveness to lentiviral transduction among various hippocampal cell types. In particular, we show for the first time that mature neurons of the granule cell layer do not express lentivirus-delivered transgenes, despite successful expression in other hippocampal cell types. Therefore, amongst hippocampal granule cells, only adult-generated neurons are target for lentivirus-mediated transgene delivery. These properties make lentiviral vectors excellent systems for overexpression or knockdown of genes in neuronal progenitor cells, immature neurons and adult-generated neurons of the mouse hippocampus in vivo.
Figures






References
-
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. - PubMed
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
-
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

