Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation
- PMID: 19144650
- PMCID: PMC2652333
- DOI: 10.1074/jbc.M806893200
Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation
Abstract
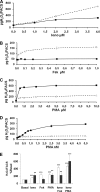
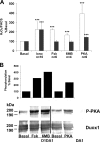
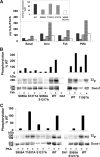
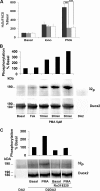
Dual oxidases were initially identified as NADPH oxidases producing H(2)O(2) necessary for thyroid hormone biosynthesis. The crucial role of Duox2 has been demonstrated in patients suffering from partial iodide organification defect caused by bi-allelic mutations in the DUOX2 gene. However, the Duox1 function in thyroid remains elusive. We optimized a functional assay by co-expressing Duox1 or Duox2 with their respective maturation factors, DuoxA1 and DuoxA2, to compare their intrinsic enzymatic activities under stimulation of the major signaling pathways active in the thyroid in relation to their membrane expression. We showed that basal activity of both Duox isoenzymes depends on calcium and functional EF-hand motifs. However, the two oxidases are differentially regulated by activation of intracellular signaling cascades. Duox1 but not Duox2 activity is stimulated by forskolin (EC(50) = 0.1 microm) via protein kinase A-mediated Duox1 phosphorylation on serine 955. In contrast, phorbol esters induce Duox2 phosphorylation via protein kinase C activation associated with high H(2)O(2) generation (phorbol 12-myristate 13-acetate EC(50) = 0.8 nm). These results were confirmed in human thyroid cells, suggesting that Duox1 is also involved in thyroid hormonogenesis. Our data provide, for the first time, detailed insights into the mechanisms controlling the activation of Duox1-2 proteins and reveal additional phosphorylation-mediated regulation.
Figures



References
-
- Dupuy, C., Ohayon, R., Valent, A., Noel-Hudson, M. S., Deme, D., and Virion, A. (1999) J. Biol. Chem. 274 37265–37269 - PubMed
-
- De Deken, X., Wang, D., Many, M. C., Costagliola, S., Libert, F., Vassart, G., Dumont, J. E., and Miot, F. (2000) J. Biol. Chem. 275 23227–23233 - PubMed
-
- Lambeth, J. D. (2004) Nat. Rev. Immunol. 4 181–189 - PubMed
-
- Babior, B. M. (1999) Blood 93 1464–1476 - PubMed
-
- Banfi, B., Clark, R. A., Steger, K., and Krause, K. H. (2003) J. Biol. Chem. 278 3510–3513 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

