Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice
- PMID: 19144689
- PMCID: PMC2670639
- DOI: 10.1152/ajprenal.90548.2008
Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice
Abstract
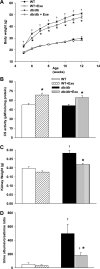
Diabetic nephropathy, the leading cause of end-stage renal disease, is characterized by a proapoptotic and prooxidative environment. The mechanisms by which lifestyle interventions, such as exercise, benefit diabetic nephropathy are unknown. We hypothesized that exercise inhibits early diabetic nephropathy via attenuation of the mitochondrial apoptotic pathway and oxidative damage. Type 2 diabetic db/db and normoglycemic wild-type mice were exercised for an hour everyday at a moderate intensity for 7 wk, following which renal function, morphology, apoptotic signaling, and oxidative stress were evaluated. Exercise reduced body weight, albuminuria, and pathological glomerular expansion in db/db mice independent of hyperglycemic status. Changes in renal morphology were also related to reduced caspase-3 (main effector caspase in renal apoptosis), caspase-8 (main initiator caspase of the "extrinsic" pathway) activities, and TNF-alpha expression. A role for the mitochondrial apoptotic pathway was unlikely as both caspase-9 activity (initiator caspase of this pathway) and expression of regulatory proteins such as Bax and Bcl-2 were unchanged. Kidneys from db/db mice also produced higher levels of superoxides and had greater oxidative damage concurrent with downregulation of superoxide dismutase (SOD) 1 and 3. Interestingly, although exercise also increased superoxides, there was also upregulation of multiple SODs that likely inhibited lipid (hydroperoxides) and protein (carbonyls and nitrotyrosine) oxidation in db/db kidneys. In conclusion, exercise can inhibit progression of early diabetic nephropathy independent of hyperglycemia. Reductions in caspase-3 and caspase-8 activities, with parallel improvements in SOD expression and reduced oxidative damage, could underlie the beneficial effects of exercise in diabetic kidney disease.
Figures



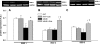

References
-
- Al-Kateb H, Boright AP, Mirea L, Xie X, Sutradhar R, Mowjoodi A, Bharaj B, Liu M, Bucksa JM, Arends VL, Steffes MW, Cleary PA, Sun W, Lachin JM, Thorner PS, Ho M, McKnight AJ, Maxwell AP, Savage DA, Kidd KK, Kidd JR, Speed WC, Orchard TJ, Miller RG, Sun L, Bull SB, Paterson AD. Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes 57: 218–228, 2008. - PMC - PubMed
-
- Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J 17: 908–910, 2003. - PubMed
-
- Baba K, Minatoguchi S, Sano H, Kagawa T, Murata I, Takemura G, Hirano T, Ohashi H, Takemura M, Fujiwara T, Fujiwara H. Involvement of apoptosis in patients with diabetic nephropathy: a study on plasma soluble Fas levels and pathological findings. Nephrology (Carlton) 9: 94–99, 2004. - PubMed
-
- Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhausl W. High-glucose-triggered apoptosis in cultured endothelial cells. Diabetes 44: 1323–1327, 1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

