Mengovirus-induced rearrangement of the nuclear pore complex: hijacking cellular phosphorylation machinery
- PMID: 19144712
- PMCID: PMC2655543
- DOI: 10.1128/JVI.01456-08
Mengovirus-induced rearrangement of the nuclear pore complex: hijacking cellular phosphorylation machinery
Abstract
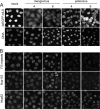
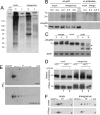
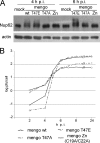
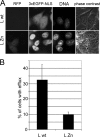
Representatives of several picornavirus genera have been shown previously to significantly enhance non-controllable bidirectional exchange of proteins between nuclei and cytoplasm. In enteroviruses and rhinoviruses, enhanced permeabilization of the nuclear pores appears to be primarily due to proteolytic degradation of some nucleoporins (protein components of the pore), whereas this effect in cardiovirus-infected cells is triggered by the leader (L) protein, devoid of any enzymatic activities. Here, we present evidence that expression of L alone was sufficient to cause permeabilization of the nuclear envelope in HeLa cells. In contrast to poliovirus, mengovirus infection of these cells did not elicit loss of nucleoporins Nup62 and Nup153 from the nuclear pore complex. Instead, nuclear envelope permeabilization was accompanied by hyperphosphorylation of Nup62 in cells infected with wild-type mengovirus, whereas both of these alterations were suppressed in L-deficient virus mutants. Since phosphorylation of Nup62 (although less prominent) did accompany permeabilization of the nuclear envelope prior to its mitotic disassembly in uninfected cells, we hypothesize that cardiovirus L alters the nucleocytoplasmic traffic by hijacking some components of the normal cell division machinery. The variability and biological significance of picornaviral interactions with the nucleocytoplasmic transport in the infected cells are discussed.
Figures



References
-
- Agol, V. I. 2002. Picornavirus genome: an overview, p. 127-148. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, DC.
-
- Beck, M., F. Förster, M. Ecke, J. M. Plitzko, F. Melchior, G. Gerisch, W. Baumeister, and O. Medalia. 2004. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science 3061387-1390. - PubMed
-
- Belov, G. A., A. G. Evstafieva, O. V. Mikitas, A. B. Vartapetian, and V. I. Agol. 2000. Early alteration of nucleocytoplasmic traffic induced by some RNA viruses. Virology 275244-248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

