Striatal versus hippocampal representations during win-stay maze performance
- PMID: 19144741
- PMCID: PMC2666400
- DOI: 10.1152/jn.91106.2008
Striatal versus hippocampal representations during win-stay maze performance
Abstract
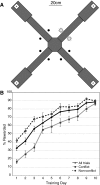
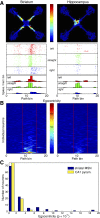
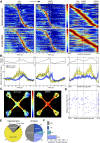
The striatum and hippocampus are widely held to be components of distinct memory systems that can guide competing behavioral strategies. However, some electrophysiological studies have suggested that neurons in both structures encode spatial information and may therefore make similar contributions to behavior. In rats well trained to perform a win-stay radial maze task, we recorded simultaneously from dorsal hippocampus and from multiple striatal subregions, including both lateral areas implicated in motor responses to cues and medial areas that work cooperatively with hippocampus in cognitive operations. In each brain region, movement through the maze was accompanied by the continuous sequential activation of sets of projection neurons. Hippocampal neurons overwhelmingly were active at a single spatial location (place cells). Striatal projection neurons were active at discrete points within the progression of every trial-especially during choices or following reward delivery-regardless of spatial position. Place-cell-type firing was not observed even for medial striatal cells entrained to the hippocampal theta rhythm. We also examined neural coding in earlier training sessions, when rats made use of spatial working memory to guide choices, and again found that striatal cells did not show place-cell-type firing. Prospective or retrospective encoding of trajectory was not observed in either hippocampus or striatum, at either training stage. Our results indicate that, at least in this task, dorsal hippocampus uses a spatial foundation for information processing that is not substantially modulated by spatial working memory demands. By contrast, striatal cells do not use such a spatial foundation, even in medial subregions that cooperate with hippocampus in the selection of spatial strategies. The progressive dominance of a striatum-dependent strategy does not appear to be accompanied by large changes in striatal or hippocampal single-cell representations, suggesting that the conflict between strategies may be resolved elsewhere.
Figures


References
-
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol 68: 945–960, 1992. - PubMed
-
- Becker JT, Walker JA, Olton DS. Neuroanatomical bases of spatial memory. Brain Res 200: 307–320, 1980. - PubMed
-
- Berke JD Procedural learning: striatum. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford: Academic, 2009.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

