Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat
- PMID: 19144754
- PMCID: PMC2665846
- DOI: 10.1152/ajpregu.90690.2008
Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat
Abstract
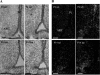
In the adult brain, leptin regulates energy homeostasis primarily via hypothalamic circuitry that affects food intake and energy expenditure. Evidence from rodent models has demonstrated that during early postnatal life, leptin is relatively ineffective in modulating these pathways, despite the high circulating levels and the presence of leptin receptors within the central nervous system. Furthermore, in recent years, a neurotrophic role for leptin in the establishment of energy balance circuits has emerged. The precise way in which leptin exerts these effects, and the site of leptin action, is unclear. To provide a detailed description of the development of energy balance systems in the postnatal rat in relation to leptin concentrations during this time, endogenous leptin levels were measured, along with gene expression of leptin receptors and energy balance neuropeptides in the medial basal hypothalamus, using in situ hybridization. Expression of leptin receptors and both orexigenic and anorexigenic neuropeptides increased in the arcuate nucleus during the early postnatal period. At postnatal day 4 (P4), we detected dense leptin receptor expression in ependymal cells of the third ventricle (3V), which showed a dramatic reduction over the first postnatal weeks, coinciding with marked morphological changes in this region. An acute leptin challenge robustly induced suppressor of cytokine signaling-3 expression in the 3V of P4 but not P14 animals, revealing a clear change in the location of leptin action over this period. These findings suggest that the neurotrophic actions of leptin may involve signaling at the 3V during a restricted period of postnatal development.
Figures




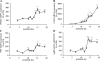


References
-
- Embryonic vertebrate central nervous system: revised terminology. The Boulder Committee. Anat Rec 166: 257–261, 1970. - PubMed
-
- Adam CL, Moar KM, Logie TJ, Ross AW, Barrett P, Morgan PJ, Mercer JG. Photoperiod regulates growth, puberty and hypothalamic neuropeptide and receptor gene expression in female Siberian hamsters. Endocrinology 141: 4349–4356, 2000. - PubMed
-
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol 62: 413–437, 2000. - PubMed
-
- Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept 92: 1–7, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

