A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy
- PMID: 19144837
- PMCID: PMC6664949
- DOI: 10.1523/JNEUROSCI.5295-08.2009
A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy
Abstract
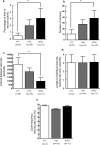
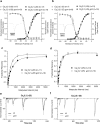
Low-voltage-activated, or T-type, calcium (Ca(2+)) channels are believed to play an essential role in the generation of absence seizures in the idiopathic generalized epilepsies (IGEs). We describe a homozygous, missense, single nucleotide (G to C) mutation in the Ca(v)3.2 T-type Ca(2+) channel gene (Cacna1h) in the genetic absence epilepsy rats from Strasbourg (GAERS) model of IGE. The GAERS Ca(v)3.2 mutation (gcm) produces an arginine to proline (R1584P) substitution in exon 24 of Cacna1h, encoding a portion of the III-IV linker region in Ca(v)3.2. gcm segregates codominantly with the number of seizures and time in seizure activity in progeny of an F1 intercross. We have further identified two major thalamic Cacna1h splice variants, either with or without exon 25. gcm introduced into the splice variants acts "epistatically," requiring the presence of exon 25 to produce significantly faster recovery from channel inactivation and greater charge transference during high-frequency bursts. This gain-of-function mutation, the first reported in the GAERS polygenic animal model, has a novel mechanism of action, being dependent on exonic splicing for its functional consequences to be expressed.
Figures



References
-
- Adams PJ, Garcia E, Snutch TP, Spacey SD. Splice variant composition of P/Q-type calcium channels affects both biophysical properties and sensitivity to an FHM point mutation. Biophys J Abstr. 2007;2869:602A.
-
- Broicher T, Kanyshkova T, Meuth P, Pape HC, Budde T. Correlation of T-channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci. 2008;39:384–399. - PubMed
-
- Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
