Functional and morphological differences among intrinsically photosensitive retinal ganglion cells
- PMID: 19144848
- PMCID: PMC2752349
- DOI: 10.1523/JNEUROSCI.4117-08.2009
Functional and morphological differences among intrinsically photosensitive retinal ganglion cells
Abstract
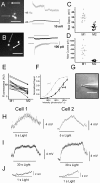
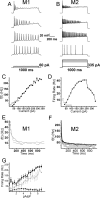
A subset of ganglion cells in the mammalian retina express the photopigment melanopsin and are intrinsically photosensitive (ipRGCs). These cells are implicated in non-image-forming visual responses to environmental light, such as the pupillary light reflex, seasonal adaptations in physiology, photic inhibition of nocturnal melatonin release, and modulation of sleep, alertness, and activity. Morphological studies have confirmed the existence of at least three distinct subpopulations of ipRGCs, but studies of the physiology of ipRGCs at the single cell level have focused mainly on M1 cells, the dendrites of which stratify solely in sublamina a (OFF sublamina) of the retinal inner plexiform layer (IPL). Little work has been done to compare the functional properties of M1 cells to those of M2 cells, the dendrites of which stratify solely in sublamina b (ON sublamina) of the IPL. The goal of the current study was to compare the morphology, intrinsic light response, and intrinsic membrane properties of M1 and M2 cells in the mouse retina. Here we demonstrate additional morphological differences between M1 and M2 cells as well as distinct physiological characteristics of both the intrinsic light responses and intrinsic membrane properties. M2 cells displayed a more complex dendritic arborization and higher input resistance, yet showed lower light sensitivity and lower maximal light responses than M1 cells. These data indicate morphological and functional heterogeneity among ipRGCs.
Figures


References
-
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. - PubMed
-
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
-
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. - PubMed
-
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
